
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
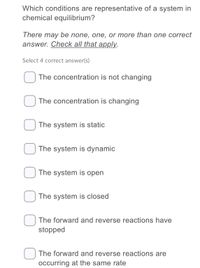
Transcribed Image Text:Which conditions are representative of a system in
chemical equilibrium?
There may be none, one, or more than one correct
answer. Check all that apply.
Select 4 correct answer(s)
The concentration is not changing
The concentration is changing
The system is static
The system is dynamic
The system is open
The system is closed
The forward and reverse reactions have
stopped
The forward and reverse reactions are
occurring at the same rate
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following statements is FALSE: Group of answer choices The value of the equilibrium constant depends on the temperature Pure solids are not included in the equilibrium constant expression. At equilibrium, the reaction has stopped At equilibrium, the concentrations of all species are constant At equilibrium, the forward and reverse reactions are happening at the same ratearrow_forwardAccording to Le Chatelier's principle, changes in concentration will induce stress on a system at equilibrium. How will the reaction shift to relieve stress induced by increases and decreases in concentration. Select all that apply. Select 2 correct answer(s) Decreasing the concentration of one substance causes the reaction to shift away from the side containing the substance that was removed. Increasing the concentration of one substance causes the reaction to shift towards the side containing the substance that was added. Decreasing the concentration of one substance causes the reaction to shift towards the side containing the substance that was removed. | Increasing the concentration of one substance causes the reaction to shift away from the side containing the substance that was added.arrow_forwardSelect all of the correct statements about equilibrium from the choices below.At equilibrium the rates of forward and reverse reactions are equal. As a reaction proceeds forward toward equilibrium the forward rate drops. At equilibrium all reactions stop. Reactants are transformed into products even at equilibrium. As a reaction proceeds forward toward equilibrium the reverse rate constant rises.As a reaction proceeds forward toward equilibrium the forward rate constant drops.arrow_forward
- Question 6 Which of the following would increase the rate of a chemical reaction? Decreasing the temperature Decreasing the surface arca of the reactants O Decreasing the concentration of the reactants O Decreasing the magnitude of "k O None of the abovearrow_forwardSelect all of the correct statements about equilibrium from the choices below. At equilibrium the rates of forward and reverse reactions are equal. As a reaction proceeds backwards toward equilibrium the reverse rate drops. As a reaction proceeds forward toward equilibrium the reverse rate rises. At equilibrium the speed of a reaction equals its rate constant. As a reaction proceeds backwards toward equilibrium the forward rate rises. At equilibrium the forward rate equals zero. Xarrow_forwardWhat is the importance of the Haber process? Group of answer choices An example of theoretical equilibrium applied to control industrial synthesis of fertilizer. A method of determining an equilibrium constant used in agriculture. A method of determining equilibrium directionality for explosives. An example of a manufacturing process to convert an equilibrium system to a non-equilibrium system. A process to determine the amount of reactant and products for equilibrium systems used in engineering.arrow_forward
- IPse_assessment_id=_2032637_1&course Remaining Time: 1 hour, 28 minutes, 28 seconds. * Question Completion Status: O Decreasing the amount of H2. QUESTION 2 *Which of the following statements about a system at chemical equilibrium is true? O The forward and reverse reactions both stop. O The forward reaction has a faster rate than the reverse reaction. O The reverse reaction has a faster rate than the forward reaction. The forward reaction rate is equal to the reverse reaction rate. O There are no more reactants left. QUESTION 3 Which statement about organic compouunds is falee? Click Save and Submit to save and submit. Click Save All Answers to save all answers. search DELL prt sarrow_forwardSelect all of the correct statements about equilibrium from the choices below. At equilibrium the rates of forward and reverse reactions are equal. At equilibrium the speed of a reaction equals its rate constant. At equilibrium concentrations of reactants and products stay constant. At equilibrium the reverse rate equals zero. ☐ At equilibrium the rate of change of product concentration is zero. As a reaction proceeds backwards toward equilibrium the product concentrations drop.arrow_forwardCombination Reactions solid magnesium metal + oxygen gas ® solid magnesium oxide solid copper metal + oxygen gas ® solid copper(II) oxide solid aluminum metal + iodine solid ® aluminum iodidearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY