
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Only typed explanation of all three subparts otherwise leave it
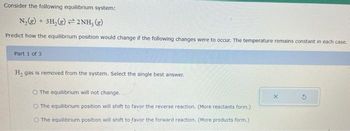
Transcribed Image Text:Consider the following equilibrium system:
N₂(g) + 3H₂(g)2NH, (g)
Predict how the equilibrium position would change if the following changes were to occur. The temperature remains constant in each case.
Part 1 of 3
H₂ gas is removed from the system. Select the single best answer.
O The equilibrium will not change.
O The equilibrium position will shift to favor the reverse reaction. (More reactants form.)
O The equilibrium position will shift to favor the forward reaction. (More products form.)
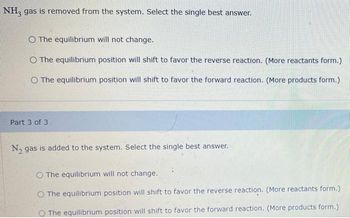
Transcribed Image Text:NH3 gas is removed from the system. Select the single best answer.
O The equilibrium will not change.
O The equilibrium position will shift to favor the reverse reaction. (More reactants form.)
O The equilibrium position will shift to favor the forward reaction. (More products form.)
Part 3 of 3
N₂ gas is added to the system. Select the single best answer.
O The equilibrium will not change.
O The equilibrium position will shift to favor the reverse reaction. (More reactants form.)
O The equilibrium position will shift to favor the forward reaction. (More products form.)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- * 60% iPad 2:23 PM + openvellum.ecollege.com Search Textbook Solutions | Chegg.com 61%iPad2:20 PM+openvellum.ecollege.comSearch... Course Home My Courses KOnline homework for CH08 Course Home Chapter 8 Multiple-Choice Problem 45 > 35 of 41 Syllabus Scores Review | Constants I Periodic Table еТext Document Sharing User Settings Part A Course Tools The process of crenation occurs when the concentration outside a cell is and is said to be higher in concentration; hypotonic higher in concentration; hypertonic lower in concentration; hypertonic lower in concentration; hypotonic Request Answer Submit Pearson Copyright O 2019 Pearson Education Inc. All rights reserved.| Terms of Use | Privacy Policy Permissions Contact Usarrow_forwardMaps G Google c Compounds i Which has the highest boiling point? Multiple Choice .docx xternal_browser=0&launchUrl=https Grammarly My Citation Lists | E... C Solved: Chapter 12... diesel fuel (composed mostly of C15H32-C18H38) gasoline (composed mostly of C5H12-C12H26) kerosene (composed mostly of C12H26-C16H34) All of these are petroelum products, so all have the same boiling point. < Prev 18 of 4 O O Darrow_forwardO Course Home b Details | bartleby O X -> A openvellum.ecollege.com/course.html?courseld=16561285&OpenVellumHMAC=dd25775cc7b85cec3204f753df519590#10001 Q * E Apps O Maps E Connect - To Do As... O ocCC Moodle P chem work b help A Balance Chemical E. Gmail YouTube CHEM-1123-EW40S Late Spring 2021 Sign Out Help Hi, ashlee v Mastering Chemistry Course Home O My Courses Syllabus Part A Scores Identify which of the following are carbohydrates. Check all that apply. еТext • View Available Hint(s) Document Sharing Но User Settings CH, Course Tools > H CH-O, H OH H C-C OH H OH OH H H O H-Ö -NH, H. ОН ОН ОН H H H H-C- -C С—С—ОН ОН ОН ОНн O H O HO-C- -C-C-OH ОН ОН ОН 10:39 PM P Type here to search 99 3/10/2021arrow_forward
- (129) ALEKS: Calculating x + X www-awu.aleks.com/alekscgi/x/isl.exe/10_u-IgNsikr7j8P3JH-IvTqeviKFP6W0cqJcWJdIACROQwyw24GWHInCwZsOvg6oljUPZmqCsVfxIjXe3ye87. ☆ 17.4 Solubility and...18.3 Gibbs Free E.. 5.3 Enthalples of... 18.5 Gibbs Free E... Reading Schedule 19.6 Reduction Po... Y SOLUTION: The le Math 115 W-S Fall... A ALEKS-Lara Althawadi X C calculating the solubility of an X esc E O KINETICS AND EQUILIBRIUM Calculating the solubility of an ionic compound when a commo... sp Calculate the solubility at 25 °C of CuBr in pure water and in a 0.0150M CoBr₂ solution. You'll find K, data in the ALEKS Data tab. Round both of your answers to 2 significant digits. 0- solubility in pure water: solubility in 0.0150 M CoBry solution: Explanation 30 Methods for Measuring the pH of an Aqueous Slide 28 of 104 Notes Q A Z Check 2 Comments 9 == W Click to add notes S 3 C с E D 4 C R P -111+ 00 0° F 0.9 0x0 5 G Search or type URL X T 63% X EX G 6 B Y 3 010 7 H ☆ Ⓒ2022 McGraw Hill LLC. All Rights…arrow_forwardInbo (534) Conv I Balar b Ansv Post CHE 101 C X с Chec bartl bartl The b My C Unkr O Sear E I ma G what app.101edu.co/# Bryant's Gmail Cascadia Canvas Lo... T GSBA Scholarship L... HOMEGROWN TRA... Learn Touch Typing... C The Science of Well... Investor360° ® Login ClickUp Reading list >> Question 38 of 40 Submit Determine the mass of solid NaCH:COO that must be dissolved in an existing 500.0 mL solution of 0.200 M CH:COOH to form a buffer with a pH equal to 5.00. The value of Ka for CH:COOH is 1.8 × 10-5. 1 2 3 NEXT > Let x represent the original concentration of CH:COO- in the water. Based on the given values, set up the ICE table in order to determine the unknown. CH:COOH(aq) + H2O(I) H:O*(aq) + CH:COO (aq) Initial (M) Change (M) Equilibrium (M) 5 RESET 0.200 5.00 -5.00 1.0 x 10-9 -1.0 x 10-9 1.0 x 10-5 -1.0 x 10-5 1.8 x 10-5 -1.8 x 10-5 x + 5.00 х- 5.00 x + 1.0 x 10 9 х- 1.0 x 109 x + 1.0 × 10-5 х- 1.0 х 105 x + 1.8 x 10-5 х- 1.8 х 10-5 + 11:01 PM e Type here to search 59°F 后 W…arrow_forwardC ?? Darrow_forward
- .arrow_forwardHelp 100% 4A To Public Health Ch x * HSC 258 - Major Projec X MindTap - Cengage Lea X C The Illustration To TH d%=55750828934189288909969212&elSBN=9781305657571&id=D1061392007&nbld=21... * Q Search th References Use the References to access important values if needed for this question. For the following reaction, 24.7 grams of sulfur dioxide are allowed to react with 9.95 grams of oxygen gas . sulfur dioxide(g) + oxygen(g) → sulfur trioxide(g) What is the maximum mass of sulfur trioxide that can be formed? grams What is the FORMULA for the limiting reagent? grams What mass of the excess reagent remains after the reaction is complete? Submit Answerarrow_forwardB File Paste E + Home Cut E Copy 4 Page 1 of 1 Clipboard Format Painter Insert 0 words S Draw Design Layout References Calibri (Body) BIU U abe 11 À A Aa A A abe X₂ X² Font Type here to search P English (Philippines) Accessibility: Investigate Mailings 10 E•E•S• Review View Help HE e W Document1 - Word €¶ Paragraph 1-32. A bottle of concentrated aqueous sulfuric acid, labeled 98.0 wt% H₂SO4, has a concentration of 18.0 M. (a) How many milliliters of reagent should be diluted to 1.000 L to give 1.00 M H₂SO4? (b) Calculate the density of 98.0 wt% H₂SO4- S ■ Tell me what you want to do AaBbCcDc AaBbCcDc AaBbC AaBbCct AaB AaBbcct AaBbCcD Normal 1 No Spac... Heading 1 Subtitle Subtle Em... Heading 2 Title Styles Sign in 1:31 (Ctrl) - ENG S IX Share Find ab Replace Select Editing 8:09 pm 18/08/2022 ▸► + 228%arrow_forward
- Please type this, dont write it on a paper.arrow_forwardthis was incorrect, im confusedarrow_forwardexe/lo_u-IgNslkr7j8P3ji Qs_dp5pR4ENzvdYC-70kXyMz36BqJhw3sVP80GQVcR8H8EYh1OU3pQMbCr ourse Home Login | Student Veri... Logout 5 MyProgrammingLab Imported From IE O ITE MEASUREMENT AND MATYER Predicting and naming ionic compounds formed by two elements Decide whether each pair of elements in the table below will form an ionic compound. If t formed in the spaces provided. empirical formula of ionic compound element #1 element #2 Forms ionic compound? name of ionic compound lithium lodine yes O no calcium sulfur Oyes no fluorine sulfur yes cesium uniseube O yes noarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY