
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
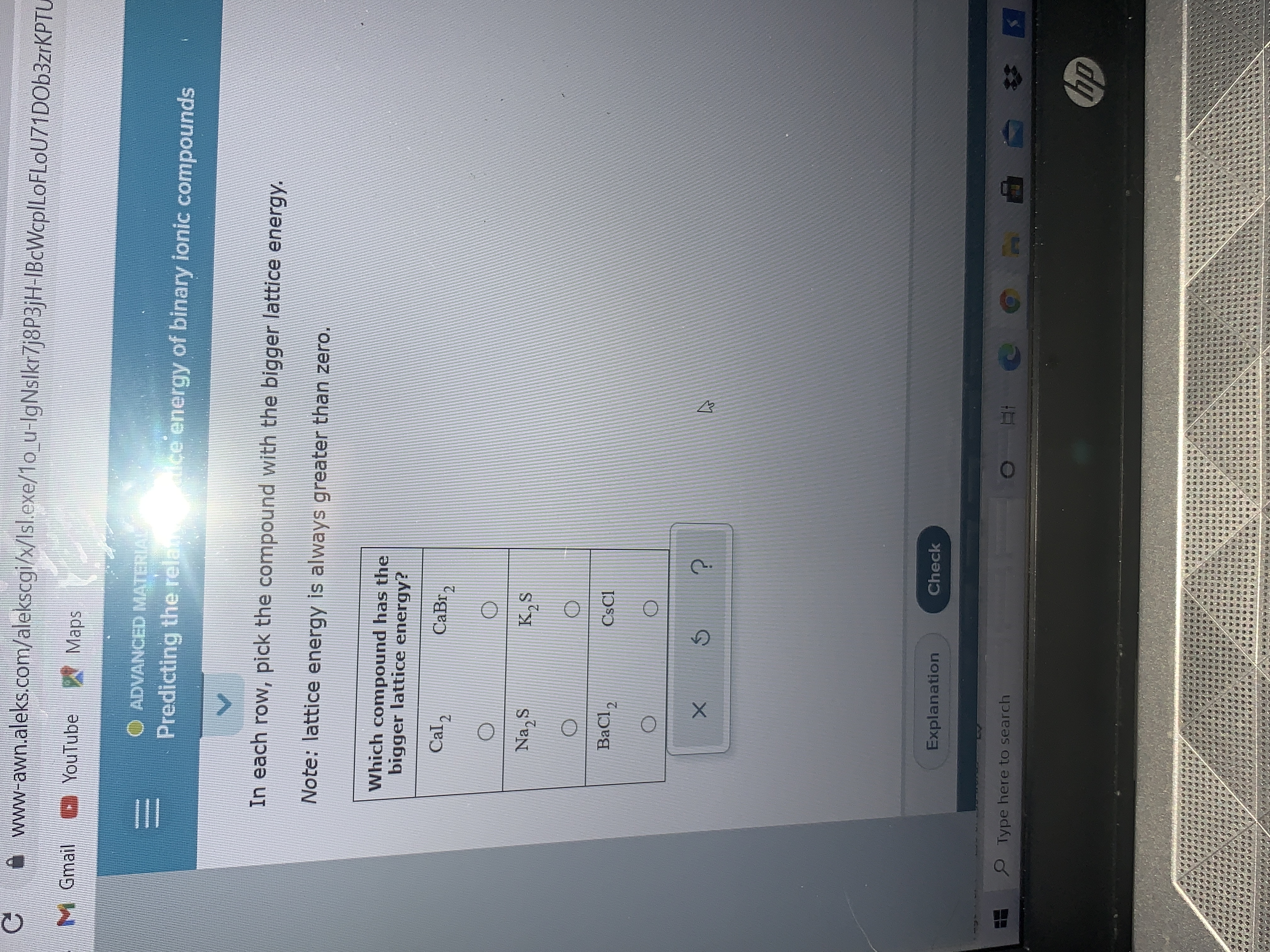
Transcribed Image Text:Which compound has the
bigger lattice energy?
Cal2
CaBr,
Na, S
BaCl,
CsCl
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the ionic compounds below would be expected to have the highest lattice energy? MgO KF LiF RbBrarrow_forwardV, PIER the compound with the bigger lattice energy. Note: lattice energy is always greater than zero. Which compound has the bigger lattice energy? MgBr2 SrBr2 Cs,0 CsF KF KIarrow_forwardWhich of the following ionic compounds has the largest lattice energy? Select an answer and submit. Forkeyboard navigation, use the up/down arrow keys to select an answer. a CaCl, b. CaO LiBr LICI e LiFarrow_forward
- 8arrow_forwarduppose a chemist discovers a new metallic element and names it "Xiguum" (Xi).Xi exhibits chemical behaviour similar to an alkaline earth. Xi(s) + Cl2(g) → XiCl2(s) Lattice energy for XiCl2 -1900. kJ/mol First Ionization energy of Xi 400. kJ/mol Second Ionization energy of Xi 680. kJ/mol Electron affinity of Cl -348.7 kJ/mol Bond energy of Cl2 239 kJ/mol Enthalpy of sublimation (atomization) of Xi 150. kJ/mol Use the above data to calculate ΔH°f for Xiguum chloride.arrow_forwardThe charges and sizes of the ions in an ionic compound affect the strength of the electrostatic interaction between the ions and thus the strength of the lattice energy of the ionic compound. Arrange the compounds according to the magnitudes of their lattice energies based on the relative ion charges and sizes. Highest lattice energy Lowest lattice energy Answer Bank KCI MgO NaF MgF₂arrow_forward
- Predicting the relative lattice energy of binary ionic compounds In each row, pick the compound with the bigger lattice energy. Note: lattice energy is always greater than zero. Which compound has the bigger lattice energy? BaCh BaS Bel₂ KF O BeBr₂ CsF O 1/5arrow_forwardUsing average bond enthalpies (linked above), estimate the enthalpy change for the following reaction: 2HBr(g) + Cl₂(9) 2HCI(g) + Br₂(g) kJarrow_forwardUsing average bond enthalpies (linked above), estimate the enthalpy change for the following reaction: CH4(g) + Cl₂(g)-CH3CI(g) + HCl(g) kjarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY