
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Please provide explanation!
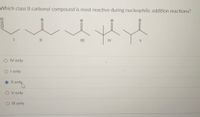
Transcribed Image Text:### Question: Reactivity of Class II Carbonyl Compounds
**Which class II carbonyl compound is most reactive during nucleophilic addition reactions?**
The image presents five structural formulae, labeled I to V, representing different class II carbonyl compounds. Each structure consists of a carbon-oxygen double bond (the carbonyl group) with varying alkyl groups attached. The compounds are arranged as follows:
1. **Compound I**: A straight chain aldehyde.
2. **Compound II**: Another straight chain aldehyde with slightly different substitution.
3. **Compound III**: A straight chain ketone.
4. **Compound IV**: A ketone with a branching methyl group.
5. **Compound V**: A ketone with a different branching pattern.
The question asks to identify which compound is most reactive in nucleophilic addition reactions.
**Options:**
- ○ IV only
- ○ I only
- ● II only (Selected)
- ○ V only
- ○ III only
**Selection Explanation:**
Compound II is highlighted as the most reactive, suggesting it likely has structural features that increase electrophilicity, such as less steric hindrance or higher inductive effects facilitating nucleophilic attack.

Transcribed Image Text:**Class II Carbonyls**
- Ketones & aldehydes
- Contains poor leaving groups (LVGs) such as R and H bonded to the carbonyl carbon
- Undergo nucleophilic addition reactions
**Diagram Description:**
1. **Ketone Structure:**
- Shows a carbonyl group (C=O) with the carbon atom bonded to two substituents, an unspecified group (R) and an amine group (NR₂).
2. **Aldehyde Structure:**
- Displays a carbonyl group (C=O) where the carbon atom is bonded to an unspecified group (R) and a hydrogen atom (H).
These diagrams illustrate the structural differences between ketones and aldehydes, highlighting their bonding patterns and tendency to undergo nucleophilic addition reactions due to their carbonyl groups.
Expert Solution
arrow_forward
Step 1
Here option 1 is aldehyde and all other are ketones.
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY