
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
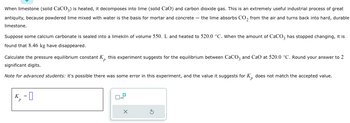
Transcribed Image Text:When limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great
antiquity, because powdered lime mixed with water is the basis for mortar and concrete - the lime absorbs CO₂ from the air and turns back into hard, durable
limestone.
Suppose some calcium carbonate is sealed into a limekiln of volume 550. L and heated to 520.0 °C. When the amount of CaCO3 has stopped changing, it is
found that 8.46 kg have disappeared.
Calculate the pressure equilibrium constant K, this experiment suggests for the equilibrium between CaCO3 and CaO at 520.0 °C. Round your answer to 2
significant digits.
P
Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K does not match the accepted value.
0
X
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At 30 degrees Celsius the Kc for 2CO(g) + O2(g) = 2CO2(g) is 2.24 x 1022. Predict in which direction the reaction will proceed to reach equilibrium, if we start with 55.0 g CO, 95.0 g O2 and 75.0 g CO2 in a 5.0 L vessel.arrow_forwardThe airbags that protect people in car crashes are inflated by the extremely rapid decomposition of sodium azide, which produces large volumes of nitrogen gas. 1. Write a balanced chemical equation, including physical state symbols, for the decomposition of solid sodium azide (NaN,) into solid sodium and gaseous dinitrogen. alb Ar 2. Suppose 12.0 L of dinitrogen gas are produced by this reaction, at a temperature of 13.0 °C and pressure of exactly 1 atm. Calculate the mass of sodium azide that must have reacted. Round your answer to 3 significant digits. Explanation Check 2021 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy Accessibility <. lenovo dle WebEx at 3pm (dia' just before 3pm) Resp L-650-479-3208 Access Code (meeting Number): 737 129 915 Dois, 3134arrow_forwardThe Haber Process, developed by Fritz Haber in 1909, was a revolutionary method for producing ammonia from elemental hydrogen and nitrogen on an industrial scale. Write a balanced equation showing the conversion from elemental nitrogen (N2) and hydrogen (H2) to ammonia (NH3). (Omit states-of-matter from your answer.)arrow_forward
- Because carbon and silicon are both elements in group 14 on the periodic table, we expect them to react with other elements in similar ways. To some extent, they do, but in some cases, carbon and silicon compounds that seem to have analogous structures have very different chemical characteristics. For example, carbon tetrachloride, CCl4, is very stable in the presence of water, but silicon tetrachloride, SiCl4, reacts quickly with water. The unbalanced equation for this reaction is SiCl4 + H2O → Si(OH)4 + HCl Balance this equation. Write a conversion factor that could be used to convert between moles of SiCl4 and moles of H2O. How many moles of SiCl4 react with 24 moles of water? Write a conversion factor that could be used to convert between moles of Si(OH)4 and moles of water. How many moles of Si(OH)4 form when 4.01 moles of H2O react with an excess of SiCl4?arrow_forwardUpon heating 134 g MgSO4 · 7 H2O:(a) how many grams of water can be obtained? (b) how many grams of anhydrous compound can be obtained?arrow_forwardWhat is the CORRECT equilibrium constant expression for the reaction of N2O (g) and O2(g) to produce NO(g)?arrow_forward
- Ancient Romans built often out of bricks and mortar. A key ingredient in their mortar was quicklime (calcium oxide), which they produced by roasting limestone (calcium carbonate). 1. Write a balanced chemical equation, including physical state symbols, for the O-0 decomposition of solid calcium carbonate (CaCO3) into solid calcium oxide and gaseous carbon dioxide. x10 Ar 2. Suppose 86.0L of carbon dioxide gas are produced by this reaction, at a temperature of 230.0 °C and pressure of exactly 1 atm. Calculate the mass of calcium carbonate that must have reacted. Be sure your answer has the correct number of significant digits.arrow_forwardThe extraction of aluminum metal from the aluminum hydroxide in bauxite ore by the Hall-Héroult process is one of the most remarkable success stories of late 19th century practical chemistry, turning aluminum from a rare and precious metal into the cheap commodity it is today. In the first step, aluminum hydroxide reacts to form alumina and water: 2 Al(OH)3(s)-Al₂O3(s) + 3 H₂O(g) In the second step, alumina and carbon react to form aluminum and carbon dioxide: 2 Al₂O3(s)+3 C(s)-4 Al(s) + 3 CO₂(g) Write the net chemical equation for the production of aluminum from aluminum hydroxide and carbon. Be sure your equation is balanced. Explanation Check 80 E5 ローロ X On F6 S MacBook Air F7 DII FB FO A Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Access F10 A F11 ola Ar 00arrow_forward6arrow_forward
- Hydrogen peroxide, H₂O₂, has a density of 1.45 g/cm³ and decomposes spontaneously but slowly. The decomposition reaction will proceed at a faster rate if iodide ion, a catalyst, is added. The steps for the catalyzed reaction are: H₂O₂(aq) + (aq) → H₂O(l) + Ol-(aq) OO H₂O₂(aq) + Ol(aq) → H₂O(l) + O₂(g) +1-(aq) a. What is the net reaction? b. If 10.00 mL of H₂O₂ undergoes this reaction, what mass of oxygen is produced? c. If the reaction has an 85.5% yield, what mass of oxygen is actually produced?arrow_forwardConsider the reaction: N2(g) + 3H2(g) 2NH3(g) where Kc = 0.500 at 400 °C. If 50.0 L reaction vessel contains 1.000 mole N₂; 3.000 mole H₂ and 0.050 mole NH3, which of the following is TRUE? The reaction based on the following parameters is in equilibrium. More ammonia will be produced as the reaction approaches equilibrium. Data provided is not enough to warrant a conclusion regarding equilibrium. More ammonia will dissociate as the reaction approaches equilibrium.arrow_forwardA 1.00-L flask was filled with 2.14 mol gaseous SO2 and 2.14 mol gaseous NO2 and heated. After equilibrium was reached, it was found that 1.55 mol gaseous NO was present. Assume that the reaction SO2 (g) + NO, (g) = SO3(g) + NO(g) occurs under these conditions. Calculate the value of the equilibrium constant, K, for this reaction. HOW DO WE GET THERE? What are the equilibrium concentrations of SO2, NO2, and SO3? [SO2] = [NO2] = M [SO3] = Marrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY