
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
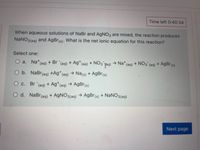
Transcribed Image Text:Time left 0:40:34
When aqueous solutions of NaBr and AgNO3 are mixed, the reaction produces
NaNO3(aq)
and AgBr(s). What is the net ionic equation for this reaction?
Select one:
O a. Na*(aq) + Br (aq) + Ag*(aq) + NO3 Ta)
→ Na* (aq) + N03 (aq) + AgBr(s)
O b. NaBr(aq) +Ag*(aq) → Na(s) + AgBr(s)
O c. Br (aq) + Ag*(aq) → AgBr(s)
O d. NaBr(aq) + AgNO3(aq) → AgBr(s) + NANO3(aq)
Next page
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- write the balanced net ionic equation Pb(NO3)2(aq)+BaCl2(aq)---PbCl2(s)+Ba(NO3)2(aq)arrow_forwardA chemist performs a gravimetric analysis. The chemist combines 1.00 L of 2.00 M AGNO, (ag) with 1.00 L of 4.00M NaCl (ag) in an Erlenmeyer flask. Both the AgNO3 (aq) solution and the NaCl(aq) solution are colorless. After the mixture has been stirred, a cloudy white substance is observed at the bottom of the flask. What is the expected mass of AgCl (s), in grams, assuming that the yield is 100%? In the box, enter the mass to the nearest gram.arrow_forwardWhat is the correct net ionic equation for the following double displacement reaction? Mg(NO3)2 (ag) +2 KF (ag) MgF (s) + 2 KNO, (ag) → a. Mg+2 (ag) + F (ag) M9F2 (s) b. Mg*2 (aq) +2 NO, (ag) + F (aq) → MgF, (s) +2 NO, (aq) c. Mg*2 (ag) + 2F (ag) - MgF2 (s) d. Mg*2 (ag) + (aq) - MgF, (s) c. K* (ag) + NO, (ag) KNO, (aq)arrow_forward
- A sample of iron ore is dissolved in acid, and the iron ore is converted to Fe. The sample is then titrated with 57.30 ml or 0.03240 M MnO solution. The oxidation-reduction reaction that occurs during titration is as follows MnO4 (aq) 5 Fe2 (aq) + 8 H (aq) - Mn (ac) SFfe (aq) 4H0 How many grams of iron were in the sample? Select one: O a. 7.631 g O b. 0.5386 g O c. 0.7631 g O d. 5.185 x 101g O e. 2.310 x 101 garrow_forwardNeed help with this chemistryarrow_forwardWrite the net ionic equation for the reaction when solutions of magnesium sulfide (MgS) and copper (1) nitrate (CUNO3) are mixed given the reaction below: Reaction: MgS (aq) + 2 CuNO3 (aq) → Mg(NO3)2(aq) + Cu₂S (s) Be sure to write out the complete ionic reaction first to find which ions are spectator ions.arrow_forward
- A useful application of oxalic acid is the removal of rust, Fe2O3. Fe2O3(s) + 6 H2C2O4(aq) → 2 Fe(C2O4)3(aq) + 3 H2O + 6 H+(aq) Calculate the number of grams of rust that can be removed by 500 mL of a 0.100M oxalic acid.arrow_forward2. If Dr. Dahm wants 450 mL of a solution that has a concentration of 0.357 M HOI (aq). Determine how many grams of ICl are needed. ICI + H₂O(1) H* (aq) + Cl(aq) + HOI(aq)arrow_forwardBased on the balanced chemical equation shown below, determine the concentration (M) of Fe2+(aq) if 40.00 mL of the Fe2+(aq) solution is required to completely react with 43.47 mL of a 0.125 M bromate, BrO3-(aq), solution. The chemical equation for the reaction is 6 Fe2+(aq) + BrO3-(aq) + 6 H+(aq) → 6 Fe3+(aq) + Br-(aq) + 3 H2O(l) Do not type in units nor write in scientific notation.arrow_forward
- A sample of iron ore is dissolved in acid, and the iron is converted to Fe2*. The sample is then titrated with 47.20 mL of 0.02240 M MnO4 solution. The oxidation-reduction reaction that occurs during titration is as follows: MnO4(aq) + 5FE2*(aq) + 8H*(aq) → Mn²*(aq) + 5FE3* (aq) + 4H2O How many moles of MnO4¯ were added to the solution? Blank 1 2+ How many moles of Fe were in the sample? Blank 2 How many grams of iron were in the sample? Blank 3 If the sample had a mass of 0.8890 g, what is the percentage of iron in the sample? Blank 4 Blank 1 Add your answer Blank 2 Add your answer Blank 3 Add your answer Blank 4 Add your answerarrow_forwardWhat is the balanced net ionic chemical equation between Cu^2+ (aq) and I^- (aq)?arrow_forwardA chemist wishes to precipitate iron ions out of a solution of iron (III) nitrate. Complete balanced equation, total ionic equation and a net ionic equation have been provided. Identify a spectator ion. Complete Balanced Chemical Equation Fe (NO3)3 (aq) + 3 NaOH (aq) --> Fe (OH)3 (s) + 3 NaNO3 (aq) Total Ionic Equation Fe3+ (aq) + 3 NO3- (aq) + 3Na+ (aq) + 3 OH- (aq) --> Fe (OH)3 (s) + 3 Na+ (aq) + 3 NO3- (aq) Net Ionic Equation Fe3+ (aq) + 3 OH- (aq) --> Fe (OH)3 (s) Identify a spectator ion:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY