
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please don't provide handwritten solution ...
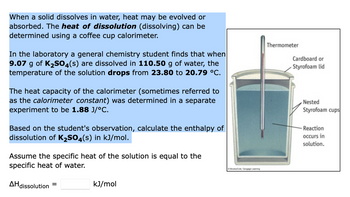
Transcribed Image Text:When a solid dissolves in water, heat may be evolved or
absorbed. The heat of dissolution (dissolving) can be
determined using a coffee cup calorimeter.
In the laboratory a general chemistry student finds that when
9.07 g of K₂SO4(s) are dissolved in 110.50 g of water, the
temperature of the solution drops from 23.80 to 20.79 °C.
The heat capacity of the calorimeter (sometimes referred to
as the calorimeter constant) was determined in a separate
experiment to be 1.88 J/°C.
Based on the student's observation, calculate the enthalpy of
dissolution of K₂SO4(s) in kJ/mol.
Assume the specific heat of the solution is equal to the
specific heat of water.
Brook, Cengage Leaming
Thermometer
Cardboard or
Styrofoam lid
Nested
Styrofoam cups
-Reaction
occurs in
solution.
AH dissolution
=
kJ/mol
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Carbon dioxide dissolved in water to yield carbonic acid. Carbonic acidthen partially dissociates to produce the hydrogen carbonate anion and ahydrogen cation. (write two equations for this step)arrow_forwardWhat molarity of HCl stock solution is needed so that 12.5 mL of this stock solution diluted to 0.500L will yield 5mM?arrow_forwardGive detailed Solution..don't give Handwritten answer..don't use Ai for answering thisarrow_forward
- An aqueous solution of aluminum iodide, All3, contains 12.6 grams of aluminum iodide and 17.5 grams of water. The percentage by mass of aluminum iodide in the solution is %.arrow_forwardCombustion of hydrocarbons such as undecane (C,H) produces carbon dioxide, a "greenhouse gas. Greenhaluse gases in the Earth's atmosahere tan trap the Sun's heat, raising the averege temperature of the Earth. For this reason there has been a great deal of international discussion about whether to regulate the production of carban diaxide. 1. Write a balanced chemical equation, including physical state symbols, for the combustion of liquid undecane into pmequs carbon diocide and gaeous water. D-0 2. Suppose 0.300 kg of undecane are burned in air at a pressure of exatly I atm nd a temperature of 17.0 "C. Calculate the volume of carbon dionide gas that is produced. Round your answer to 3 significant digits.arrow_forwardHow many mL of a 0.203 M aqueous solution of cobalt(II) nitrate, , must be taken to obtain 6.24 grams of the salt?arrow_forward
- What can you reasonably predict to occur when elemental bromine is added to a solution containing the iodide ion? Write chemical equations as part of your explanation.arrow_forwardHow do I calculate/ what is the equation for the % weight/weight when I have a mass in grams and a volume of titrant delivered? Unknown Chrloride mass 0.1173g and 35.47 ml.arrow_forwardPlease don't provide handwritten solution ...arrow_forward
- 4.4 Discuss the process of sampling and preparation of laboratory sample in quantitative analysis.arrow_forwardThe mass of K3PO4 needed to prepare 250.0mL of an aqueous solution in which PO4-3 concentration is 0.0550M. The answer is ……………………… 6arrow_forwardplease use the information in the photo attached to calculate the molarity of the students sodium hydroxide solution, round to 3 significant digits.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY