
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Please don't provide handwritten solution.....
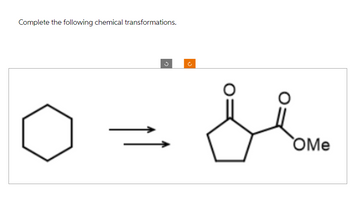
Transcribed Image Text:Complete the following chemical transformations.
OMe
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- [References] Use the References to access important values if needed for this question. In the laboratory, a student dilutes 17.8 mL of a 9.01 M hydroiodic acid solution to a total volume of 200.0 mL. What is the concentration of the diluted solution? AM 3 Concentration = Submit Answer M Try Another Version 1 item attempt remaining Cengage Learning Cengage Technical Support Previousarrow_forwardMass of sodium is 22.99, oxygen 16.75g hydrogen 1.21g how many grams of NaOH are in 305 ml of a .6M solution please use correct sig figsarrow_forwardPlease don't provide handwritten solution .....arrow_forward
- Please don't provide handwritten solution...arrow_forwardWhat volume (mL) of 0.2875 M NaOH is theoretically required to titrate 20.00 mL of 0.1276 M HCl? What is the percent error, if the volume of 0.2875 M NaOH that is experimentally required to titrate 20.00 mL of 0.1276 M HCl is found to be 11.84 mL?arrow_forwardPlease don't provide handwritten solution .....arrow_forward
- Please anwer question and exlpain whyarrow_forwardTrue/False Indicate whether the statement is true or false. Write T if the statement is true while F if statement is false on the before each number 1.During inspection, the attributes and variable characteristics of a product is being compared against its specification to find out if the product is within the prescribed limits 2.Standard solutions of acids should be preserved in tightly stoppered bottle fitted with soda-lime tube to protect from carbon dioxide of the air 3.All burets should be tested for leakage prior to use Aarrow_forwardAt a particular temperature, suppose that 24.4 g solute is added to 50.0 g water and, after mixing, 0.3 g of the solute remains undissolved. What is the solubility of this solute at this temperature in grams per 100 g of water? (Enter in standard notation with one digit after the decimal point) Type your answer......arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY