
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
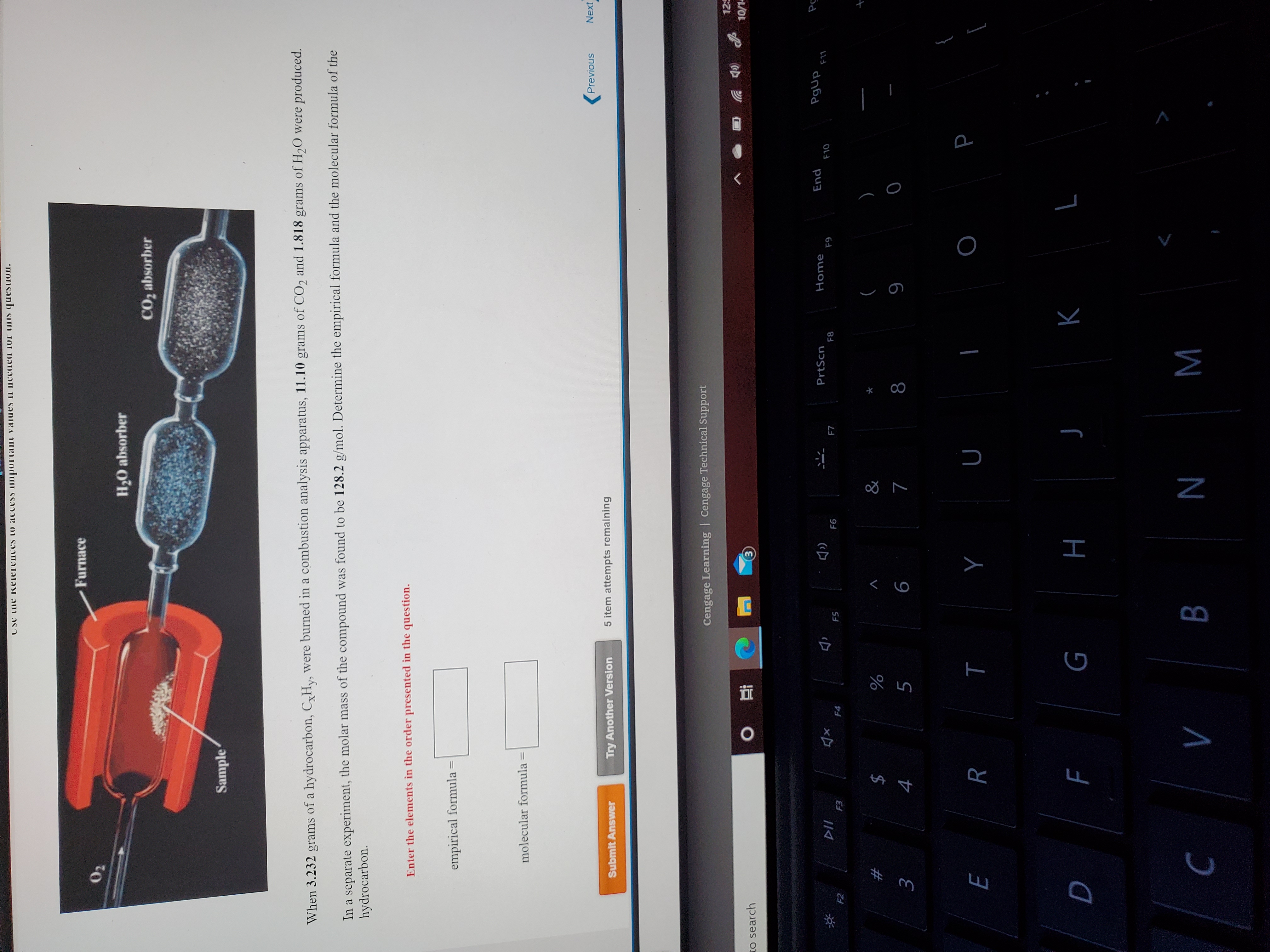
Transcribed Image Text:When 3.232 grams of a hydrocarbon, C,Hy, were burned in a combustion analysis apparatus, 11.10 grams of CO2 and 1.818 grams of H2O were produced.
In a separate experiment, the molar mass of the compound was found to be 128.2 g/mol. Determine the empirical formula and the molecular formula of the
hydrocarbon.
Expert Solution
arrow_forward
Step 1
Data given:
Mass of sample = 3.232 g
Mass of CO2 = 11.10 g
Mass of H2O = 1.818 g
Molar mass of the compound = 128.2 g/mol
Step by stepSolved in 8 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When octane, C8H18, is burned in the presence of excess O2, the usual products of carbon dioxide, CO2, and water, H2O, form. Write a balanced chemical equation for this reaction and calculate the grams of H2O produced from the combustion of 7.90×102 g C8H18.arrow_forwardComplete combustion of 4.50 g of a hydrocarbon produced 14.4 g of CO2 and 5.15 g of H2O. What is the empirical formula for the hydrocarbon?arrow_forwardAniline, a starting material for urethane plastic foams, consists of C, H, and N. Combustion of such compounds yields CO2, H2O, and N2 as products. If the combustion of 9.71 g of aniline yields 6.63 g H2O and 1.46 g N2, determine the empirical formula of aniline and the molecular formula, if the molar mass of aniline is 93 g/mol.arrow_forward
- Consider the reaction shown below: HCO3-1 (aq) + OH-1 (aq) ⇋⇋ CO3-2(aq) + H2O (l) Identify the Brønsted-Lowry acid in this reaction. Select one: a. HCO3-1 b. OH-1 c. CO3-2 d. H2Oarrow_forwardA compound has an empirical formula of CH2. An independent analysis gave a value of 70 g/mol for its molar mass. What is the molecular formula of the compound?arrow_forwardAn Unknown compound contains only carbon, hydrogen, nitrogen, and oxygen (C, H, N, & O). Complete combustion of 3.54 g of pure compound in pure oxygen produced 8.49 g CO2, 2.14 g H2O, and 0.300 g N2. What is the empirical formula for benzocaine?arrow_forward
- Alcoholic fermentation converts glucose (C6H12O6) into ethanol (C2H5OH) and carbon dioxide (CO2). What mass of carbon dioxide can be formed from the alcoholic fermentation of 31.6 g of glucose?arrow_forwardAn unknown compound contains only C, H, and O. Combustion of 7.00 g of this compound produced 15.9 g CO2 and 6.51 g H2O. Convert the masses of C, H, and O in the unknown compound to moles.arrow_forwardC. Ethylene glycol is used as an automobile antifreeze and in the manufacture of polyester fibers. Combustion of 6.38 mg of ethylene glycol gives 9.06 mg CO₂ and 5.58 mg H₂O. The compound contains only C, H, and O. What is the empirical formula of ethylene glycol?arrow_forward
- Complete combustion of 4.5892 a particular compound that contains only carbon, hydrogen and oxygen produces 10.4324 g of CO2 and 4.2705 g of H2O. The empirical formula of the compound is given by C H O (indicate "1" if there is only one atom of a given type in the empirical formula.) The molar mass of the compound is found to be 116.16 g/mol in a different experiment. What is the molecular formula of the compound? C H Oarrow_forwardAn unknown compound has a formula CxHyOz. You burn 0.1705 g of the compound and isolated 0.4163 g of CO2 and 0.1074 g of H2O. What is the empirical formula of the compound? If the molar mass is 72.1 g/mol, what is the molecular formula.arrow_forwardAn unknown hydrocarbon, which contains only C and H, is tested by combustion analysis to determine its empirical formula. When 1.50g of this substance is completely combusted, 4.40g of CO2 and 2.70g of H2O are produced. What is the empirical formula of the unknown?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY