
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
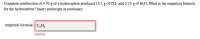
Transcribed Image Text:Complete combustion of 4.70 g of a hydrocarbon produced 15.1 g of CO2 and 5.15 g of H2O. What is the empirical formula
for the hydrocarbon? Insert subscripts as necessary.
empirical formula: ||C,H,
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- H2. Consider the following reaction: CH4 + 2O2 --> 2H2O + CO2 How many moles of water can be formed from 3.7 moles of CH4 and 3.0 moles of O2? Explain with detailsarrow_forwardKermesic acid, a red substance extracted from insects for use as a dye molecule, contains only C, H, and O. Combustion of 10.0 g of kermesic acid produces 21.33 g of CO₂ and 2.728 g of H₂O. Determine the molar mass of the compound if it is between 250.0 and 350.0 g/mol. Provide an answer to four significant figures.arrow_forwardWhen 0.86g of organic compound containing C,H and O was burned completely in oxygen 1.64g of CO2 and 1.01 g of H2O were produced. If the molecular mass of the compound is 138 g/mol determine the molecular formula C2H304. O C4H1202. C6H1803. C8H2404. C5H10014. Oarrow_forward
- Vitamin C, an antioxidant present in citrus fruits, contains only C, H, and O. Combustion of 10.0 g of vitamin C produces 14.99 g of CO₂ and 4.092 g of H₂O. Determine the molar mass of the compound if it is between 150.0 and 220.0 g/mol. Provide an answer to four significant figures.arrow_forwardEthanol (C,H O) is combusted in air according to the following reaction: C,H,O(1) + O,(g) –→ CO,(g) + H,0(I) How many grams of water would be produced by the complete combustion of 5.07 moles of ethanol in the presence of excess oxygen? + MacBook Pro 888 こニ。 esc & # $ @ 7 2 3 4 R Y Q W E ab F А lock C V option command ontrol cの つ つ エarrow_forwardhow did you get the numbers beside the balanced equations. such as 4 beside H2O and 2 beside N2.arrow_forward
- Methanol has tremendous potential to be used as an alternate fuel. Carbon monoxide gas reacted with hydrogen gas can be used to produce methanol.A student reacted 51.9 g of H2(g) with excess CO(g) to produce methanol.If his percent yield was 47.7%, calculate the mass (in g) of CH3OH(l) that he collected in the experiment.Hint: Write a balanced chemical reaction equation.Record only your final answer with the correct number of significant digits and the proper units.arrow_forwardGiven the reaction: 2C,H, + 70, → 4C0, + 6H,0 What is the total number of moles of CO, produced by the complete combustion of 5.0 moles of C,H,? 2.0mol 1.0mol 5.0mol O 10.molarrow_forwardthe reaction of 15 moles of carbon with 30 moles of oxygen will result in a theoretical yield of ____ moles of CO2 is what?arrow_forward
- 6. Consider the following chemical reaction: 2 Cr(OH)3(aq) >Cr₂O3(s) + 3H₂0(1) 12.00 moles of chromium (III) hydroxide is decomposed. Calculate moles of the water produced. O 18.00 moles O 1.500 moles O 4.00 moles O 12.00 molesarrow_forwardFor the following reaction: C3H8(g) + 02 ——-> CO2(g) +H20(g) a). balance the reaction b). determine the limiting reactant from 20.0 grams of each starting reactant c). calculate the theoretical yield of CO2(g) in grams d). calculate the percent yield if the actual yield was 10.7 gramsarrow_forwardThe combustion of 135 mg of a hydrocarbon sample produces 440. mg of CO2 and 135 mg H2O. The molar mass of the hydrocarbon sample is 270 g/mol. Determine the molecular formula of the hydrocarbon.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY