
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
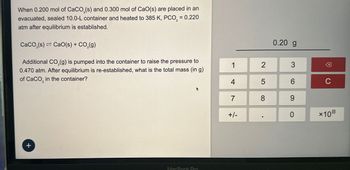
Transcribed Image Text:When 0.200 mol of CaCO3(s) and 0.300 mol of CaO(s) are placed in an
evacuated, sealed 10.0-L container and heated to 385 K, PCO, = 0.220
atm after equilibrium is established.
CaCO3(s) Cao(s) + CO2(g)
Additional CO2(g) is pumped into the container to raise the pressure to
0.470 atm. After equilibrium is re-established, what is the total mass (in g)
of CaCO in the container?
+
MacBook Pro
0.20 g
1
2
3
☑
4
5
6
C
7
8
9
+/-
0
*10
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 4 images

Knowledge Booster
Similar questions
- Steam reforming of methane ( CH, ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 2.0 L flask with 1.9 atm of methane gas and 2.1 atm of water vapor, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 4.6 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K, = 0arrow_forward"Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas will form carbon dioxide and hydrogen, and in fact this reaction is one of the ways hydrogen is made industrially. A chemical engineer studying this reaction fills a 200. mL flask with 3.6 atm of carbon monoxide gas and 2.5 atm of water vapor. When the mixture has come t equilibrium she determines that it contains 2.0 atm of carbon monoxide gas, 0.90 atm of water vapor and 1.6 atm of carbon dioxide. The engineer then adds another 1.2 atm of carbon monoxide, and allows the mixture to come to equilibrium again. Calculate the pressure of hydrogen after equilibrium is reached the second time. Round your answer to 2 significant digits. atm x10arrow_forwardWhen 0.200 mol of CaCO,(s) and 0.300 mol of CaO(s) are placed in an evacuated, sealed 10.0-L container and heated to 385 K, PCO, = 0.220 atm after equilibrium is established. 2 CACO,(s) = CaO(s) + CO,(g) Additional CO,(g) is pumped into the container to raise the pressure to 0.470 atm. After equilibrium is re-established, what is the total mass (in g) of CaCO, in the container?arrow_forward
- The equilibrium constant, K, for the following reaction is 6.30 at 723 K. 2NH3(g) ⇒N₂(g) + 3H₂(g) If an equilibrium mixture of the three gases in a 10.9 L container at 723 K contains 0.409 mol of NH3(g) and 0.291 mol of N₂, the equilibrium concentration of H₂ is M.arrow_forwardSteam reforming of methane ( CH, ) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 1.5 L flask with 3.2 atm of methane gas and 1.1 atm of water vapor, and when the mixture has come to equilibrium measures the partial pressure of hydrogen gas to be 2.0 atm. Calculate the pressure equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. K, = Iarrow_forwardGive detailed solutionarrow_forward
- Consider the following reaction:COCl2(g) CO(g) + Cl2(g) If 1.64×10-3 moles of COCl2(g), 0.398 moles of CO, and 0.360 moles of Cl2 are at equilibrium in a 18.2 L container at 843 K, the value of the equilibrium constant, Kc, is .arrow_forwardA student ran the following reaction in the laboratory at 696 K: 2HI(g) H2(g) + I2(g) When he introduced HI(g) at a pressure of 3.40 atm into a 1.00 L evacuated container, he found the equilibrium partial pressure of HI(g) to be 2.68 atm. Calculate the equilibrium constant, Kp, he obtained for this reaction. Kp =arrow_forward"Synthesis gas" is a mixture of carbon monoxide and water vapor. At high temperature synthesis gas will form carbon dioxide and hydrogen, and in fact this reaction is one of the ways hydrogen is made industrially. A chemical engineer studying this reaction fills a 200. mL flask with 2.3 atm of carbon monoxide gas and 4.0 atm of water vapor. When the mixture has come to equilibrium he determines that it contains 0.90 atm of carbon monoxide gas, 2.6 atm of water vapor and 1.4 atm of carbon dioxide. The engineer then adds another 1.0 atm of water, and allows the mixture to come to equilibrium again. Calculate the pressure of hydrogen after equilibrium is reached the second time. Round your answer to 2 significant digits. 0 atm x10 Xarrow_forward
- Carbon disulfide and chlorine react according to the following equation: CS2(g) + 3Ch(g) =S,Cl½(g) + CC1,(g) When 1.14 mol of CS, and 4.80 mol of Clh are placed in a 2.00-L container and allowed to come to equilibrium, the mixture is found to contain 0.650 mol of CC14. How many moles of Cl are present at on equilibrium? Select one: O a. 0.490 mol O b. 2.85 mol O c. 3.50 mol O d. 0.650 mol O e. 1.43 mol e to search F3 F4 F5 F6 FZ FB F9 F10 F11 F12 Prise %24 E R T G H K B N M 立arrow_forwardUse the References to access important values if needed for this question. The equilibrium constant, Kc, for the following reaction is 0.0290 at 1150 K. 2SO3 (g)2SO2(g) + O2(g) If an equilibrium mixture of the three gases in a 19.7 L container at 1150 K contains 0.251 mol of SO3(g) and 0.353 mol of SO2, the equilibrium concentration of O₂ is M. Submit Answer Retry Entire Group 9 more group attempts remainingarrow_forwardAn equilibrium mixture of SO2,O2,and SO3 at high temperature contains the gases at the following concentrations: SO2 =3.17*10-3 mol/L,O2=4.83*10-3 mol/L and SO3=4.50*10-3 mol/L.Calcualte the equilibrium constant,Kc,for the reaction. 2SO2(g) + O2(g)=2SO3(g) Kc= please solvearrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY