
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
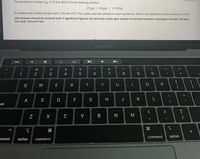
Transcribed Image Text:The equilibrium constant, Kp, is 79.9 at 2500 K for the following reaction:
XY2(g) + XZ2(g) = 2 XYZ(g)
A container was initially charged with 0.292 atm XYZ. The system was then allowed to reach equilibrium. What is the equilibrium partial pressure of XYZ?
(the answer should be entered with 3 significant figures; do not enter units; give answer in normal notation--examples include 1.23 and
12.3 and -123 and 120.)
MacBook Pro
esc
!
@
$
&
一
1
2
3
7
{
Q
W
E
T
Y
А
S
D
F
H.
J
K
ock
C
V
command
option
control
option
command
.. ..
リ
V
* CO
B
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please don't provide handwritten solution .....arrow_forwardFor the following reaction: A(g) + B(g) = C(g) Calculate the equilibrium constant K given the following information: The initial pressure of A is 4.421 bar and the initial pressure of B is 3.829 bar The equilibrium pressure of C is 1.699 bar.arrow_forwardA chemical engineer is studying the following reaction: N₂(g) + 3H₂(g) → 2 NH3(g) At the temperature the engineer picks, the equilibrium constant K for this reaction is 0.00014. The engineer charges ("fills") three reaction vessels with nitrogen and hydrogen, and lets the reaction begin. He then measures the composition of the mixture inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction vessel A B C compound N₂ H₂ NH3 N₂ H₂ NH₂ N₂ H₂ NH₂ pressure 32.70 atm 49.38 atm 23.45 atm 33.09 atm 50.55 atm 22.67 atm 33.10 atm 50.59 atm 22.64 atm expected change in pressure ↑ increase ↑ increase ↑ increase ↑ increase ↑ increase O ↑ increase ↑ increase ↑ increase ↑ increase decrease ↓ decrease ↓ decrease ↓ decrease ↓ decrease decrease ↓ decrease decrease decrease (no change) (no change) (no change) (no change) (no change) O (no change) (no…arrow_forward
- The formation of nitrogen monoxide from its elements has an equilibrium which favors reactants: N2 (g) + 02 (g) 2 NO (g) K, = 0.0290 at 2400°C If the initial partíal pressures of both reactant gases are 0.717 atm, what will be the equilibrium partial pressure of nitrogen monoxide? (A certain simplification is valid in solving this problem). 0.112 atm (A) (B) 0.0290 atm (C) 0.688 atm (D) 0.0563 atm (E) 0.0611 atmarrow_forwardAt 1000 K, a sample of pure NO2 gas decomposes: 2NO₂(g) 2NO(g) + O₂(g) The equilibrium constant, Kp, is 158. Analysis shows that the partial pressure of O₂ is 0.19 atm at equilibrium. Calculate the pressure of NO and NO₂ in the mixture.arrow_forwardConsider the equilibrium system described by the chemical reaction below. For this reaction, Kp: 109 at 298 K. If the equilibrium partial pressures of Br2 and NOBR at 0.0159 atm and 0.0768 atm, respectively, determine the partial pressure of NO at equilibrium... %D 2 NO(g) + Br2(g) = 2 NOBr(g) 1 NEXT へ If (x) represents the equilibrium partial pressure of NO, set up the equilibrium expression for Kp to solve for the partial pressure. Do not combine or simplify terms.. Kp 109 %3D RESET (109) (0.0159) (0.0768) 2(0.0159) 2(0.0768) (0.0159)? (0.0768)² (x) (2x) (x)? (2x)?arrow_forward
- A student ran the following reaction in the laboratory at 303 K:2NO(g) + Br2(g) 2NOBr(g)When she introduced NO(g) and Br2(g) into a 1.00 L evacuated container, so that the initial partial pressure of NO was 1.15 atm and the initial partial pressure of Br2 was 0.408 atm, she found that the equilibrium partial pressure of Br2 was 0.138 atm.Calculate the equilibrium constant, Kp, she obtained for this reaction. Kp =arrow_forwardConsider the equilibrium system described by the chemical reaction below. If the partial pressures at equilibrium of NO, Cl2, and NOCI are 0.095 atm, 0.171 atm, and 0.28 atm, respectively, in a reaction vessel of 7.00 L at 500 K, what is the value of Kp for this reaction? 2 NO(g) + Cl2(g) = 2 NOCI(g)arrow_forward: Given : A (g) +B (g) 2C (g) An equilibrium mixture contains 0,5 mol of each of A and B and 3.5 mol of C in one-liter container at a certain temperature. (a) How many moles of C must be added to the con- tainer to increase the new equilibrium mole number of A to 0.75? (b) If 1 mol of each of A, B and C is added to the first equilibrium mixture, what will be the new equi- librium concentration of each species at the same temperature?arrow_forward
- Consider the following reaction that takes place at 1200 °C: H, (g) + CI, (g) + 2HCI (g) Kc = 2.5x10 Hydrogen gas and chlorine gas were added to a reaction vessel at partial temperatures of 2.00 atm and 1.00 atm, respectively. Calculate the total pressure in the flask at equilibrium.arrow_forwardA chemical engineer is studying the following reaction: H2(9)+Cl2(g) → 2 HCl(g) At the temperature the engineer picks, the equilibrium constant K, for this reaction is 1.1. The engineer charges ("fills") three reaction vessels with hydrogen and chlorine, and lets the reaction begin. He then measures the inside each vessel from time to time. His first set of measurements are shown in the table below. Predict the changes in the compositions the engineer should expect next time he measures the compositions. reaction vessel compound pressure expected change in pressure H, 2.75 atm t increase I decrease (no change) Cl, 4.00 atm t increase O I decrease O (no change) A HCI 2.10 atm O f increase OI decrease O (no change) H, 2.78 atm t increase I decrease O (no change) Cl, 4.03 atm t increase I decrease (no change) 2.06 atm t increase I decrease (no change) HCl H2 3.22 atm O f increase I decrease (no change) t increase I decrease (no change) Cl, 4.98 atm OI decrease O (no change) H CI 4.26 atm…arrow_forwardType the word True or False in the provided space for the following. Consider the following system at equilibrium where Kc = 9.52x10-2 and AH° = 18.8 kJ/mol at 350 K. CH4 (g) + CCl4 (g) → 2 CH₂Cl₂ (g) The production of CH₂Cl2 (g) is favored by: decreasing the temperature. increasing the pressure. increasing the volume. removing CH₂Cl2. removing CCl4. I. II. III.____ IV. V.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY