
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What would be the volume of 3.90 moles of an ideal gas, at STP? What is the pressure of this gas, if the temperature was increased from 0 degC to 56.9 degC as it stays in a constant volume container?
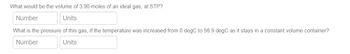
Transcribed Image Text:What would be the volume of 3.90 moles of an ideal gas, at STP?
Number
Units
What is the pressure of this gas, if the temperature was increased from 0 degC to 56.9 degC as it stays in a constant volume container?
Number
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- What is the average energy per molecule of a gas at 592 K? unit J %3D Assuming the gas is 02, has a molecular mass of 32 u, what is the rms speed of each molecule? unitarrow_forwardYou have a container of neon (Ne) gas at 290 K. The volume of the container is 0.1 m3 and the pressure is 2.1 atm. a) How many Ne atoms are in the container? b) How many moles of Ne are in the container?arrow_forwardWhat is the RMS speed of Helium atoms when the temperature of the Helium gas is 312.0 K? (Possibly useful 1.66x10-27 kg, Boltzmann's constants: the atomic mass of Helium is 4.00 AMU, the Atomic Mass Unit is: 1 AMU constant is: kg = 1.38×10-23 J/K.) kB Submit Answer Tries 0/12 What would be the RMS speed, if the temperature of the Helium gas was doubled? Submit Answer Tries 0/12 =arrow_forward
- Problem 3. The viral coefficients of a gas at 20 °C and 11.5 bar are B = -138 cm³ mol¹ and C=7222 cmº mol². Calculate the V (molar volume) Z (compressibility factor) of the gas. Use the equation below (R = 83.14 cm³ bar mol-¹ K-¹). PV 2 = ² = (1 + = + =) Z RTarrow_forwardA sealed 99 m3 tank is filled with 6000 moles of ideal oxygen gas (diatomic) at an initial temperature of 270 K. The gas is heated to a final temperature of 320 K. The atomic mass of oxygen is 16.0 g/mol. The mass density of the oxygen gas, in Sl units, is closest to:arrow_forward2.40 moles of an ideal gas is initially at 40.0 oC and 1.35 atm. a) What is its volume under these conditions?b) If it is then compressed to one third of its initial volume and its pressure increases to 2.0 atm, what, in Centigrade degrees, will be its temperature?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON