
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
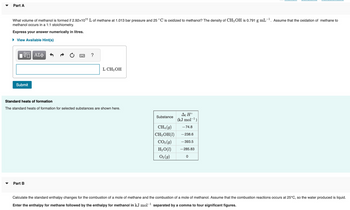
Transcribed Image Text:Part A
What volume of methanol is formed if 2.92x1011 L of methane at 1.013 bar pressure and 25 °C is oxidized to methanol? The density of CH3OH is 0.791 g mL-¹. Assume that the oxidation of methane to
methanol occurs in a 1:1 stoichiometry.
Express your answer numerically in litres.
View Available Hint(s)
VO ΑΣΦ
Submit
?
Part B
L CH3OH
Standard heats of formation
The standard heats of formation for selected substances are shown here.
Substance
CH₁ (9)
CH3OH (1)
CO₂(g)
H₂O(1)
O₂(g)
Af Ho
(kJ mol-¹)
-74.8
-238.6
-393.5
-285.83
0
Calculate the standard enthalpy changes for the combustion of a mole of methane and the combustion of a mole of methanol. Assume that the combustion reactions occurs at 25°C, so the water produced is liquid.
Enter the enthalpy for methane followed by the enthalpy for methanol in kJ mol-¹ separated by a comma to four significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How many moles of gas must be forced into a 3.1 LL ball to give it a gauge pressure of 8.4 psipsi at 23 ∘C∘C? The gauge pressure is relative to atmospheric pressure. Assume that atmospheric pressure is 14.6 psipsi so that the total pressure in the ball is 23.0 psipsi .arrow_forwardDetermine the quantity of energy given 10.95 dm³ of oxygen gas at STP reacts in this equation" 3O2(g) + 4AlBr3(s) --> 2Al2O3(s) + 6Br2(l) + 90.23kJ kJ = (10.95dm³ O2 / 1) * (A/B) * (C/D) = E 1. Regarding (A/B) which statements may be used? 1 L 3 mol O2 +90.23 kJ 22.4 dm³ O2 -90.23 kJ 1 dm³ 1 mol O2 2. Regarding (C/D), what are the values of these variables?arrow_forwardaring Suppose that 2.45 g of gaseous nitrogen, N2, is placed in a 2.15 L container The pressure is measured to be 475.0 mmHg What is the Celsius temperature of the gas? [Hint: Your first step should be solving the ideal gas equation for T.] ΜΕ ΑΣΦ ?arrow_forward
- If the reaction below took place at STP, what is the volume of O2(g) required to react with 40.0 grams of ZnS(s)? Show all work and report you final answers to the correct units and significant figures. 2ZnS(s) + 3O2(g) -> 2 ZnO(s) + 2 SO2(g)arrow_forwardRecorded barometric pressure: 758.9 mmHg Mass of Aluminum used= .021 g Volume of gas in gas burette after placing into leveling tank= 23 mL temperature in levelling tank: 21 oC Room Temperature: 294.45 K Partial pressure of water vapor: 17.5 mmHg 2 Al (s)+ 6HCl (aq)>>>>2 AlCl3 (aq)+ 3 H2 (g) 1. How to calculate the theoretical yield of H2 gas (mL) based on the mass of Aluminum used. 2. How to calculate the % error of H2 gas.arrow_forwardWhat is the Qc for the following reaction, if a mixture contains gases with the following concentrations: CH4, 26 M; H2O, 24 M; CO, 26 M; H2 15 M, at a temperature of 760 0C? CH4(g) + H2O(g) CO(g) + 3H2(g)arrow_forward
- Please help, will provide helpful ratings for correct solution. (Gpt/Ai wrong ans not allowed) Q.A reaction at 18.0 °C evolves 603. mmol of sulfur tetrafluoride gas. Calculate the volume of sulfur tetrafluoride gas that is collected. You can assume the pressure in the room is exactly 1 atm. Be sure your answer has the co number of significant digits. volume: L x10 Xarrow_forward4. At 5°C and 1 atm, x L of O2 gas is required to react with 0.51 g of glucose, what is the value of x? The unbalanced reaction is shown below: C6H12O6 (s) + O2(g) → CO2(g) + H2O(l)arrow_forwardThe compressibility factor Z = PV/nRT for O2 at -38.6°C and 519.6 atm is 1.60; at 162.8°C and 105.4 atm, it is 0.99. A certain mass of oxygen occupied a volume of 2.10 L at 162.8°C and 105.4 atm. Calculate the volume occupied by the same quantity of oxygen at -38.6°C and 519.6 atm. place the answer rounded to the hundredths place Please provide only typed answer solution no handwritten solution needed allowedarrow_forward
- An 1.82g sample of a gas occupies 6.8L at a temperature of -100.∘∘C and 3.5psi. What is the identity of this gas?arrow_forwardg P1.36 A cylinder of compressed gas contains 3.21 × 10³ of N₂ gas at a pressure of 3.75 X 107 Pa and a temperature of 15.0°C. What volume of gas has been released into the atmosphere if the final pressure in the cylinder is 2.35 × 105 Pa? Assume ideal behavior and that the gas temperature is unchanged.arrow_forwardWhat is kinetic energy of one mole of an ideal gas st 25C?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY