
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:Ili
00
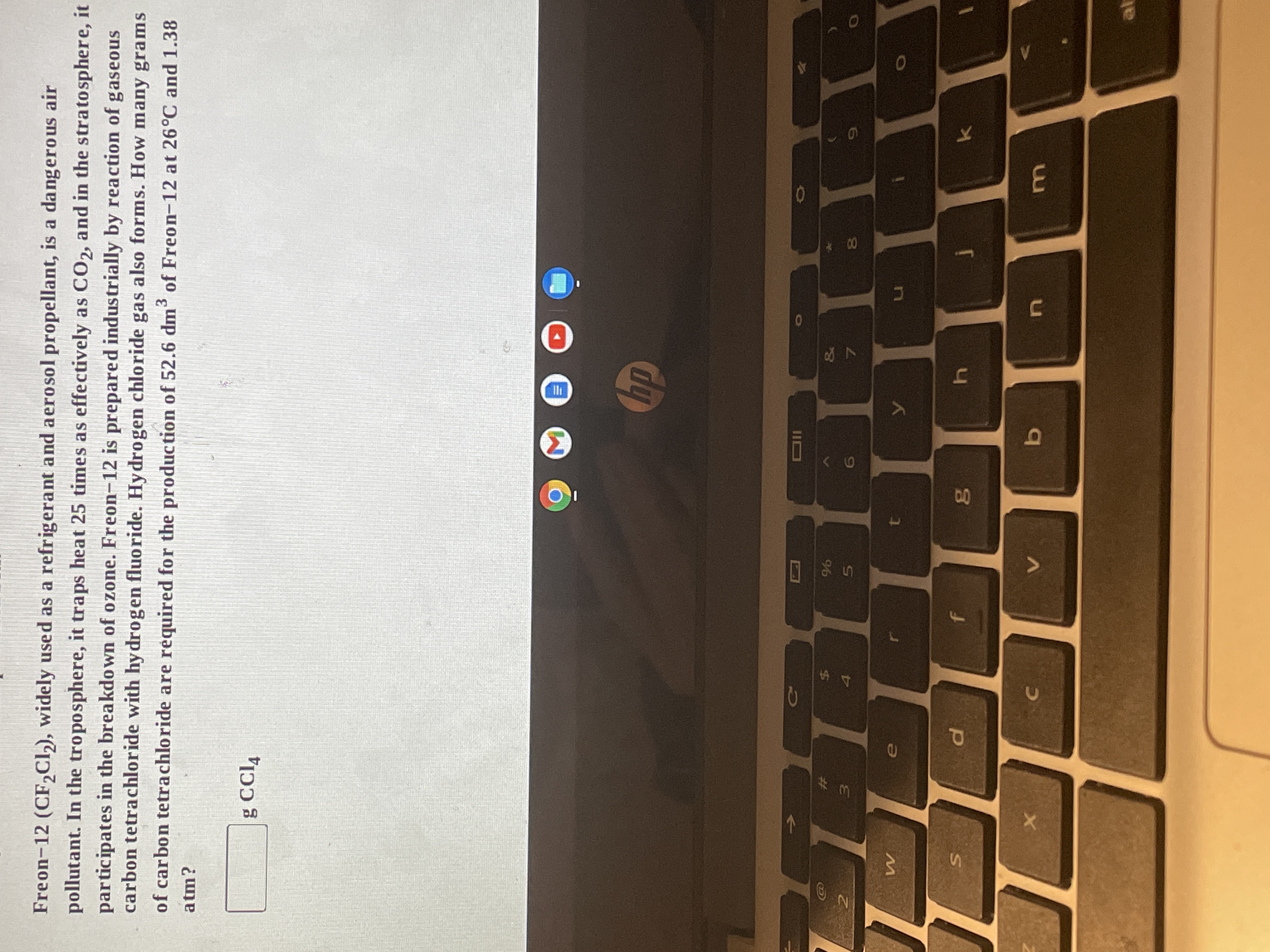
Freon-12 (CF,Cl), widely used as a refrigerant and aerosol propellant, is a dangerous air
pollutant. In the troposphere, it traps heat 25 times as effectively as CO2, and in the stratosphere, it
participates in the breakdown of ozone. Freon-12 is prepared industrially by reaction of gaseous
carbon tetrachloride with hy drogen fluoride. Hydrogen chloride gas also forms. How many grams
of carbon tetrachloride are required for the production of 52.6 dm of Freon-12 at 26°C and 1.38
atm?
g CCl,
%24
6)
(NO
n
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- To the nearest 0.1 g, how many grams of a candle wax from a different brand of candle can be burned in 5-gallon container sealed with air? Assumptions (may or may not be needed): Complete combustion to CO2 and H2O; Candle wax reacts in a ratio of 1 mol wax: 40 moles O2; MW (wax) = 370 g/mol, Air = 21% oxygen, R= 0.082 L.atm.K-1.mol-1, 1 gallon = 3.785 L, T=278 K, P = 1 atmarrow_forward(Record your answer with rounded answer to proper significant digits and the proper units.) 1.How many moles of butane (C4H10(g)) were combusted in the presence of excess oxygen gas if 55.8 L of water vapour at STP is collected?Hint: Write a balanced chemical reaction equation first. 2.One of the steps to sweeten sour gas using the Claus process is reacting hydrogen sulfide gas with sulfur dioxide gas to produce water vapour and sulfur. 16H2S(g)+8SO2(g)----->16H2O(g)+3S8(s) 8.75 kL of hydrogen sulfide at 175 kPa and 250 °C reacts with excess sulfur dioxide. Calculate the mass, in kg, of sulfur produced.arrow_forwardMass→Mass & Mass→Vol : Zinc citrate is used in toothpaste it is synthesized from zinc carbonate and citric acid according to the following reaction. A) What mass of Zn₃(C₆H₅O₇)₂ can be produced from 2240. g of ZnCO₃? B) How many L of CO₂ will be produced at STP by this amount?arrow_forward
- 5. Calculate the volume of oxygen consumed at SATP (25 °C, 100kPa) by the combustion of 10.4 kg of propane, C3H8. (arrow_forwardUpon heating 134 g MgSO4 · 7 H2O:(a) how many grams of water can be obtained? (b) how many grams of anhydrous compound can be obtained?arrow_forwardFeCl₃ has a van't Hoff factor of i = 3.40. What is the concentration of particles in a 0.501 M solution of FeCl₃?arrow_forward
- The eruption of volcano Mt. Tambora in 1816 increased Earth's albedo by 0.009525. By how much (in K) did Earth's average surface temperature decrease? Use the following expression (from Hites, page133), to account for the greenhouse effect (AE): OT4 = (1-a)/4 +AE. Assume initial values for T = 288 K, o = 5.67*10^8 Wm-2 k-4, a= 0.30, and = 1372 Wm-2arrow_forwardCalculate the pressure and composition of air on the top of Mt. Everest, assuming that the atmosphere has a temperature of 0∘C independent of altitude (h=29141ft). Assume that air at sea level is 20%O2 and 80%N2.arrow_forwardFreon-12 (CF2Cl2), widely used as a refrigerant and aerosol propellant, is a dangerous airpollutant. In the troposphere, it traps heat 25 times as effectively as CO2, and in thestratosphere, it participates in the breakdown of ozone. Freon-12 is prepared industrially byreaction of gaseous carbon tetrachloride with hydrogen fluoride. Hydrogen chloride gasalso forms. How many grams of carbon tetrachloride are required for the production of16.0 dm3 of Freon-12 at 27°C and 1.20 atm?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY