
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
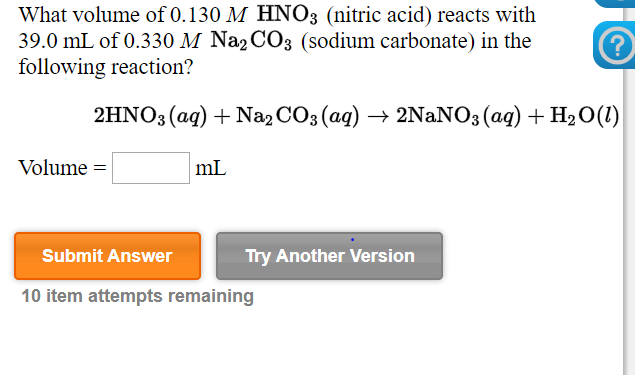
Transcribed Image Text:What volume of 0.130 M HNO3 (nitric acid) reacts with
39.0 mL of 0.330 M Na2 CO3 (sodium carbonate) in the
following reaction?
2HNO3 (ag) + Na2 CO3 (ag) → 2NANO3 (ag) + H2 0(1)
Volume =
mL
Submit Answer
Try Another Version
10 item attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Similar questions
- If 5.43 mL of a 0.52 M NaOH solution was used to neutralized the sulfuric acid in a 25.12 mL sample. Determine the molarity of the original H2SO4 solution, if NAOH and H2SO4 reaction in an Acid/Base reaction. Answer: M H2SO4 (Input only a numeric answer)arrow_forward© Macmillan Learning Nitrogen and hydrogen combine at a high temperature, in the presence of a catalyst, to produce ammonia. N₂(g) + 3 H₂(g) →→→ 2NH₂(g) Assume 0.110 mol N₂ and 0.353 mol H₂ are present initially. After complete reaction, how many moles of ammonia are produced? NH₂: How many moles of H₂ remain? H₂: How many moles of N₂ remain? به mol mol molarrow_forwardQUESTION 9 (4b-301-196-3.69) A 196 gram sample of glucose (C6H1206) was used to make a 3.69 M solution. What is the volume of that solution? Show all work and give your answer with three sig figs. Note M(C6H1206) = 180.16 g/mol. QUESTION 10 (4b-401-1.53-1) An intern working in a research lab knocks over a beaker containing an important solution. The beaker originally contained 1.50 L of a 1.53 M solution, but after it spilled it only contained 1 L of solution. The intern decides to hide his mistake by refilling the beaker to contain 1.50 L again by adding pure water (not realizing that this will dilute the solution). What is the new concentration of the solution? Show all work and give your answer with three sig figs. Click Save and Submit to save and submit. Click Save All Answers to save all answers. Save All Answers ADR 1600 4101 1O Aa MacBook Air 80 000 O00 DII DD F2 F3 F4 F5 F6 F7 F8 F9 F10 F11 %23 2.arrow_forward
- Part A - Relating Relative Numbers of Anions and Cations to Chemical Formulas If you have an aqueous solution that contains 1.5 mol of HCI, how many moles of ions are in the solution? О 10 О 15 О 20 O 25 О 30 Submit Request Answer Provide Feedbackarrow_forwardRemaining Time: 1 hour, 01 minute, 47 seconds. * Question Completion Status: QUESTION 2 The number of water molecules in 2.3 mg of water is O 6.02 x 1023 O 77.0 x 1023 O 7.7 x 1023 O 7.7 x 1019 none of the above QUESTION 3 A ctudent makes a colution bu discolvina 254a ofNDOH int0 450 gof water Wh. Click Save and Submit to save and submit. Click Save All Answers to save all answer ere to search DELLarrow_forwardYou are asked to prepare a 1.000 L solution of 4.5 M you commit a user error while preparing this solution. Assumed volume Volumetric error Preparation details Added water 2.0 cm above the line, which corresponds to 8.2 mL (0.0082 L) additional solution volume C6H12O6 (glucose; molar mass = 180.16 g/mol) in a lab by dissolving 811.0 g of glucose in water. Consider the following two scenarios in whic Prepared in a beaker Prepared in a volumetric flask 1.000 L You add the glucose to a volumetric flask and then add water until it dissolves. The water bottle you are using has a worn tip, and you inadvertently add too much water such that the meniscus is above the line. The diameter of the neck of the volumetric flask is 2.29 cm. 811.0 g glucose Concentration: You decide to evaluate and compare the errors you made while preparing the solutions using the different methods. Calculate the actual concentrations of the intended 4.5 M glucose solutions prepared by each method based on their…arrow_forward
- ogle Search S How many grams are there in 1. D How to find the Number of Aton x + Question 4 of 8 Determine the mass in grams of HCI that can react with 0.750 g of Al(OH)s according to the following reaction Al(OH):(s) + 3 HCI(aq) → AICI:(aq) + 3 H:O(aq) ADD FACTOR ANSWER RESET *( ) 78.00 0.750 6.022 x 1023 3 1.05 0.351 1 133.33 0.0288 0.117 9.60 x 10 36.46 2.25 18.02 g Al(OH); mol HCI g AICI mol AICIs mol Al(OH): g HCI mol H20 g AICIS g H:O mol AICI,arrow_forwardDilution Practice How many mL of HCI, 0.1 M, will you need to prepare 0.700 L of HCL, 0.025 M? (Ans. 175 mL of aqueous HCI, 0.1 M} Write the problem set up that justifies the answer. 1 DELL 女 23 $ & 3 6. 7 t i W e r y u d f g h jarrow_forwardIn the laboratory, a student dilutes 20.1 mL of a 6.58 M nitric acid solution to a total volume of 250.0 mL. What is the concentration of the diluted solution? Concentration = M Submit Answer Try Another Version 3 item attempts remainingarrow_forward
- Question 7 of 10 Submit What volume in L of a 0.724M Nal solution contains 0.405 | mol of Nal ? X STARTING AMOUNT ADD FACTOR ANSWER RESET *( ) %3D 0.405 0.293 g Nal L 126.90 g Nal/mol 0.559 mol Nal 559 mL 22.99 M Nal 148.89 6.022 x 1023 1.79 0.724 Tap here or pull up for additional resourcesarrow_forward(Incorrect) How many grams of AgCl are formed when 7.80 mL of 0.500 M AgNO3 is added to 6.25 mL of 0.300 M NH4CI? AgNO3(aq) + NH4Cl(aq) → AgCl(s) + NH4NO3(aq) 0.0304 g 0.822 (Your answer) 0.553 g 0.269 g (Correct answer) 1.61 garrow_forwardMixture 1 Reference 2 Extra Fe(NO3)3 3 Extra KSCN 4 Add Na₂SO3 5 Add NaCl 6 Add AgNO3 Volume (mL) | Volume (mL) of 0.002 M Fe(NO3)3 2.00 2.00 2.00 2.00 2.00 2.00 of 0.002 M Other Addition KSCN 2.00 2.00 2.00 2.00 2.00 2.00 N/A 0.2 mL of 0.1 M Fe(NO3)3 0.2 mL of 0.1 M KSCN Na₂SO3 NaCl 1 mL of 0.1 M AgNO3arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY