
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:dit
View
History
Bookmarks
Profiles
Tab
Window
Help
13- HW 16 Connect X
nheducation.com/ext/map/index.html?_con%3Dcon&external_browser%3D0&launchUrl=https%253.
Google Docs
Google Slides R URI Portals
do Poll Everywhere b Ba
Microsoft Office
Saved
5 attempts left
Check my work
Enter your answer in the provided box.
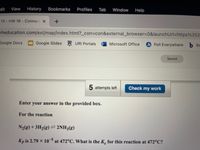
For the reaction
+3H2(g)= 2NH38)
Kp is 2.79 x 10 at 472°C. What is the K. for this reaction at 472°C?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 5arrow_forwardConsider the following equilibrium: N₂ (g) + 3H₂ (g) 2NH3 (g) AG=-34. KJ Now suppose a reaction vessel filled with 3.73 atm of nitrogen (N₂) and 1.60 atm of ammonia (NH3) at 493. °C. Answer the following questions about this system: Under these conditions, will the pressure of N, tend to rise or fall? Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of N₂ will tend to rise, can that be changed to a tendency to fall by adding H₂? Similarly, if you said the pressure of N₂ will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H₂ needed to reverse it. Round your answer to significant digits. Ⓒrise fall O yes O no atm X S ? do Ärarrow_forward101edu.co b For the gas-phase equilibrium A(g) + 2 B(g) = C(g) the initial partial pressures of A, B, and C are all 0.300 atm. After equilibrium is established at 25°C, it is found that the partial pressure of C is 0.230 atm. What is AG for this reaction? (R = 8.314 J/mol-K). 1 Q 2 W 3 E $ 4 R % 5 T Question 12 of 12 A 6 & 7 Presen 8 Home ( 9 End Carrow_forward
- The equilibrium reaction Fez O4(s) + 3 H2(g) 2 3 Fe(s) +4 H20(g) has AH° = 149.77 kJ/mol. Write down Shift Left, Shift Right, or No Change for each of the following situations: a) Solid Fe is added to the reaction. b) The temperature of the reaction is increased. c) The volume of the reaction vessel is suddenly doubled with no change in temperature. d) After doubling the volume of the reaction vessel, the partial pressure of H20 is tripled with no change in temperature.arrow_forward2. Consider the equilibrium system: 2 NOB (g) 2 NO (g) + Br2 (g) At a 298 °K, analysis of an equilibrium mixture finds the following concentrations: [Br2] = 4.0 mol/L, [NOB1] = 0.50 mol/L, [NO] = 2.0 mol/L. Calculate Keq for the reaction at this temperature. (:arrow_forwardplz given correct answer with detail step by step explanationarrow_forward
- Choose the letter of the correct answer. N204 (g) = (reversible to) 2NO2 (g), K = 4.63 x 10-3 I. the given is equation is an example of homogenous equilibrium II. Equilibrium will lie to the right Which statement(s) is/are correct?arrow_forwardN2(g) + 3H2 (g) = 2NH3 (g) AH°pn =-92 kJ Which of the following changes would cause the value of K for this reaction to increase? You can choose any combination of answers. Decreasing the volume of the reaction container. Heating the reaction container Cooling the reaction container Adding Helg) to the reaction container removing NH3(g) from reaction container.arrow_forwardAt -7.73 °C the pressure equilibrium constant K, =6.8 × 10° for a certain reaction. Here are some facts about the reaction: • The constant pressure molar heat capacity C, 1.76 J·mol -1. K¯!. • If the reaction is run at constant pressure, the volume increases by 14.%. The initial rate of the reaction is 11. mol·L - 1 -1 •S O Yes. Using these facts, can you calculate K, at 15. °C? O No. If you said yes, then enter your answer at right. Round it to 2 significant digits. Yes, and K, will be bigger. If you said no, can you at least decide whether K at Yes, and K, will be 15. °C will be bigger or smaller than K, at -7.73 °C? smaller.arrow_forward
- 3.) For the following equilibrium reaction2NO2(g) ↔N2O4(g) + Δlook in the Tro text to see what LeChatelier's principle states about gases. Assume that the reaction occurs in an enclosed movable piston. [Hint: remember that PV=nRT] a.) The pressure of the reaction is increased. b.) The volume of the reaction is decreased. c.) The volume of the reaction is increased.arrow_forwardConsider the following equilibrium: N2 (g)+3H2(g)2NH3 (g) AG = -34. kJ Now suppose a reaction vessel is filled with 2.15 atm of nitrogen (N2) and 2.19 atm of ammonia (NH3) at 236. °C. Answer the following questions about this system: OO rise ☐ x10 fall OO Under these conditions, will the pressure of NH3 tend to rise or fall? Is it possible to reverse this tendency by adding H₂? In other words, if you said the pressure of NH3 will tend to rise, can that be changed to a tendency to fall by adding H2? Similarly, if you said the pressure of NH3 will tend to fall, can that be changed to a tendency to rise by adding H₂? If you said the tendency can be reversed in the second question, calculate the minimum pressure of H2 needed to reverse it. Round your answer to 2 significant digits. yes no ☐ atm Sarrow_forwardConsider the equilibrium system described by the chemical reaction below. At equilibrium, a sample of gas from the system is collected into 5.00 L flask. The flask is found to contain 8.62 g of CO, 2.60 g of H2, 43.0 g of CH4, and 48.4 g of H:O at 320.0 °C. What are the values of Kc and Kp for this reaction? CH:(g) + H2O(g) = CO(g) + 3 H2(g) 1 2 NEXT > Based on the given data, set up the expression for Kc and then evaluate it. Do not combine or simplify terms.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY