
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
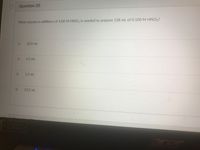
Transcribed Image Text:Question 20
What volume in milliliters of 4.00 M HNO3 is needed to prepare 128 mL of 0.100 M HNO3?
32.0 mL
3.2 mL
1.2 mL
12.5 mL
1080
arer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- nitric acid could be used to neutralize barium hydroxide. if you have 0.432 L of a 0.555 M barium hydroxide solution, how much 3.00 M nitric acid will be required to neutralize it 0.240 L 0.160 L 0.0799 L or 0.719 Larrow_forwardWhat volume of water must be added to 742 mL of a 4.5 M HCI to diluted it to 0.8 M? 4.25 L 1.65 L 4173.8 mL 3431.8 mL 3.92 Larrow_forwardHow many milliliters of a stock solution of 7.00 MM HNO3HNO3 would you have to use to prepare 0.170 LL of 0.550 MM HNO3HNO3? V= mL If you dilute 16.0 mLmL of the stock solution to a final volume of 0.350 LL , what will be the concentration of the diluted solution? M= Marrow_forward
- A chemistry student needs 10.0 ml of 0.350M NaOH. The only NaOH solution in the lab has a concentration of 5.00M. What volume of 5.0M solution must be used to prepare the needed solution? 1.43 mL 0.700 mL 0.175 mL 143 mLarrow_forwardWhat volume of 5.00 M NaOH stock solution is needed to prepare 100.0 mL of 0.250 M NaOH solution? 80.0 mL 5.00 mL 12.5 mL 0.200 mLarrow_forward1. 100.0 mL of a 0.500 M solution of KBr is diluted to 500.0 mL. What is the new concentration of the solution? 2.100.0 mL of a 0.885 M solution of KBr is diluted to 500.0 mL. What is the new concentration of the solution? 3.If 0.250 L of a 9.70 M HNO3 solution is diluted to 2.00 L, what is the molarity of the new solution?arrow_forward
- A stock solution of 1.50 M NaCl is available. How many milliliters are needed to make 3.25 L of 0.550 M NaCl solution?arrow_forward9.8 g of H2SO4 (molar mass 98 g/mol) is present in 2.0 L of solution. The molarity of the solution is: 0.010 M 0.10 M 0.050 M 0.20 M MacBook Air ►► esc 20 F1 F3 F4 F6 F7 F8 F9 F10 F2 #3 %2$ % & 2 3 5 7 8 9.arrow_forwardOrder: ABC 1.5 g. Stock: ABC 2500 mg / 2 mL. How many mL will you give? O 1 mL 0.1 mL 0.6 mL O 1.2 mLarrow_forward
- How would you prepare 207 mL of 0.214 M NaCl solution using an available 1.90 M solution? Dilute 0.0233 mL of the 0.214 M solution to a final volume of 207 mL. Dilute 23.3 mL of the 1.90 M solution to a final volume of 207 mL. Dilute 207 mL of the 0.214 M solution to a final volume of 42.9 mL. Dilute 69.9 mL of the 1.90 M solution to a final volume of 207 mL. Submit Answer Try Another Version 1 item attempt remainingarrow_forwardHow many milliliters of 2.0 M sulfuric acid are needed to make 400.0 mL of 1.00 M H 2SO 4? 0.00500 mL 200. mL 500. mL 350. mL 0.200 mLarrow_forwardIf a chemist wishes to dilute a 1.000 x 103 mg/L stock solution to prepare 5.000 × 10² mL of a working standard that has a concentration of 4.500 mg/L, what volume of the 1.000 x 10³ mg/L standard solution is needed? mLarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY