
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
What type of
Is Reaction #3 favorable under mammalian cellular conditions when the concentration of dGTP is 0.2 M, dCMP is 20 mM, dGMP-dCMP is 7 mM, and PPi is 10 mM? Use △G to support your answer. Show steps
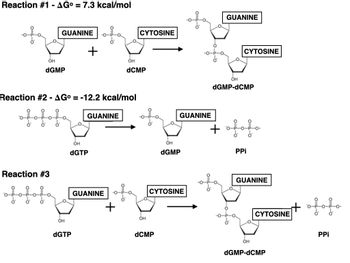
Transcribed Image Text:Reaction #1 - AG° = 7.3 kcal/mol
GUANINE
OH
dGMP
Reaction #3
ofofofo
Reaction #2 - AG° = -12.2 kcal/mol
ofofa
OH
+
dGTP
GUANINE
OH
dGTP
GUANINE
CYTOSINE
OH
dCMP
OH
dCMP
GUANINE
OH
dGMP
CYTOSINE
0=4-0
GUANINE
CYTOSINE
OH
dGMP-dCMP
+ ofofo
PPi
GUANINE
CYTOSINE
OH
dGMP-dCMP
ofofo
PPi
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 2 images

Knowledge Booster
Similar questions
- 14) Calculate K’eq and ΔG’0 for the following: A 0.1 M solution of glucose 1-phosphate at 25oC is incubated with a catalytic amount of phosphoglucomutase, the glucose 1-phosphate is transformed to glucose 6-phosphate. At equilibrium, the concentrations of the reaction components are Glucose 1-phosphate (4.5 X 10-3 M) and Glucose 6-phosphate (9.6 X 10-2 M).arrow_forwardIn a alkaline phosphatase kinetics experiments a studnet first made a standard curve with different amounts of PNP. In a reaction carried out with 10nM alkaline phosphatase and 100 uM PNPP substrate the student measured an absorbance value of 0.76 at 410nm after 30 seconds of reaction. Assuming the 30 second time point is in the lienar range of the reaction and the substrate has not been depleted at this time point, calculate the Vo at 100uM PNPP (in units of concentration per time).Beer's law formula given is y=0.0323x (y axis is Absorbance) (x Axis is PNP uM)arrow_forward5) In the below two step transformation, the first step AG is positive i.e. 1.7 kJ/mol. Yet this two step coupled reaction takes place to form Fructose-1.6-bisphosphate. Explain why?" C-H CH-OH CH,-0 H-C-OH AG"- 1.7 KJ/mol (+0.4 kcalmol) C=0 AG"=-14.2 k/mol(-3.4 kcalmol) HO-C-H HO-C-H HO-C-H H-C-OH H-C-OH H-C-OH H-C-OH H-C-OH ATP ADP H-C-OH CH-0-P. CH,-0-P-o- CH,-o- Glucose-6-phosphate Fructose-6-phosphate Fructose-1,6-bisphosphatearrow_forward
- Consider the reaction catalyzed by PFK. In the presence of AMP, which of the following will be expected? Check all that apply: a)The Km for substrate would be decreased b) the initial velocity plot would show the curve shifted to the right c) the R state is stabilized d)the rate of the reaction is diminishedarrow_forwardPaper electrophoresis of Asn, Ala, Asp, Lys and Ser mixtures at pH = 7 shows the fastest movement towards the anode The fully saponified 10g oil sample consumed 1.87g KOH, and the iodine value of the oil was 28.3. So how many unsaturated double bonds are there in each oil molecule on average? (lodine is known to have an atomic weight of 127) Given that 100g cellulose sample is completely hydrolyzed to obtain 78g glucose, then the percentage of cellulose in the sample is _% Given that the Km of catalase is 20mmol/L and the substrate concentration is 80mmol/L, then the percentage of catalase bound to the substrate is %arrow_forwardFor an enzyme kinetics experiment, a student prepared a reaction mixture by mixing 450 microliters of 0.75mM PNPP with 4.25ml of 0.2M Tris-HCl buffer. When he is ready to measure the absorbance, he added 0.3ml of Alkaline Phosphatase to the mixture and mixed thoroughly. What is the substrate concentration at the beginning of the reaction in mM ?arrow_forward
- 8L.16.2arrow_forwardA research group discovers a new version of happyase, which they call happyase*, that catalyzes the chemical reaction: HAPPY-SAD The researchers begin to characterize the enzyme. The researchers determined the kcat of the enzyme to be 452 s-1. In a separate experiment with [E;) at 1.5 nM and [HAPPY) at 38 µM, the researchers find that Vo is equal to 320 nM s-1. What is the measured Km of happyase* for its substrate HAPPY? Make sure to include units in the answer.arrow_forwardmake a Gibbs free energy graph as free energy as y-axis a for the transition state as a function of [Gdn HCl] (x-axis) with a line of best fitarrow_forward
- Can you please help show how to find Kr?arrow_forwardLysozyme catalyzes a "bi-bi" reaction, which means there are (how many) reactants and (how many) products. List, in order, the reactants that bind and the products that are released during a lysozyme-catalyzed reaction cycle -- be succinct but be specific. 1. First reactant = 2. First product = 3. Second reactant = 4. Second product %3Darrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON