
Chemistry: An Atoms First Approach
2nd Edition
ISBN: 9781305079243
Author: Steven S. Zumdahl, Susan A. Zumdahl
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Only typed explanation otherwise leave it
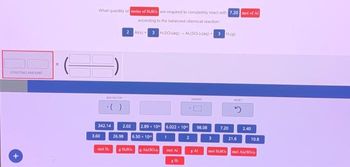
Transcribed Image Text:STARTING AMOUNT
+
X
What quantity of moles of H.SO are required to completely react with 7.20 mol of Al
according to the balanced chemical reaction:
Al(s) 3 H.SO.(aq)-Al(SO.)(aq) + 3 Hi(g)
ADD FACTOR
3.60
342.14
( )
mot H
2.02 2.89 10
26.98 6.50 10
180. Al(80)
6.022 x 10"
1
mol Al
H.
2
NEVER
98.08
BAI
3
7.20
HENT
2
21.6
2.40
10.8
mol 1.50 mol Al(50)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The pictures below show a molecular-scale view of a chemical reaction between H2 and CO to produce methanol, CH3OH The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reaction has gone to completion. D Was there a limiting reactant in this reaction? If so, what was it? Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forwardA 0.20 mol sample of magnesium burns in air to form 0.20 mol of solid MgO. What amount (moles) of oxygen (O2) is required for a complete reaction?arrow_forwardMany over-the-counter antacid tablets are now formulated using calcium carbonate as die active ingredient, which enables such tablets to also be used as dietary calcium supplements. As an antacid for gastric hyperacidity, calcium carbonate reacts by combining with hydrochloric acid found in the stomach, producing a solution of calcium chloride, converting die stomach acid to water, and releasing carbon dioxide gas (which the person suffering from stomach problems may feel as a burp). Write die balanced chemical equation for this process.arrow_forward
- 4-89 If 7.0 kg of is added to 11.0 kg of to form which reactant is in excess?arrow_forwardSmall quantities of oxygen gas can be generated in the laboratory by the decomposition of hydrogen peroxide. The unbalanced equation for the reaction is H2O2(uz/)-? H2O(/) + O2(g) Calculate the mass of oxygen produced when 10.00 g of hydrogen peroxide decomposes.arrow_forwardWrite the balanced chemical equation for the complete combustion of adipic acid, an organic acid containing 49.31% C, 6.90% H, and the remainder O, by mass.arrow_forward
- Methanol, CH3OH, is used in racing cars because it is a clean-burning fuel. It can be made by this reaction: CO(g)+2H2(g)CH3OH(l) What is the percentage yield if 5.0103gH2 reacts with excess CO to form 3.5104gCH3OH ?arrow_forwardWrite an equation from the following description: reactants are gaseous NH3 and O2, products are gaseous NO2 and liquid H2O, and the stoichiometric coefficients are 4, 7, 4, and 6, respectively.arrow_forward4.79 Phosphoric add (H3PO4) is important in the production of both fertilizers and detergents. It is distributed commercially as a solution with a concentration of about 14.8 M. Approximately 2.1 X l09 gallons of this concentrated phosphoric acid solution is produced annually in the United States. Assuming that all of this H3PO4 is produced by the reaction below, what mass of the mineral fluoruapatite (Ca5(PO4)3F) would be required each year? Ca5( PO4)3F+5H2SO43H3PO4+5CaSO4+HFarrow_forward
- 3.75 The following pictures show a molecular-scale view of a chemical reaction between the compounds AB2 and B2. (A atoms are shown in blue and B atoms in white). The box on the left represents the reactants at the instant of mixing, and the box on the right shows what is left once the reac- tion has gone to completion. Write a balanced chemical equation for this reaction. As usual, your equation should use the smallest possible whole number coefficients for all substances.arrow_forward39. Standard solutions of calcium ion used to test for water hardness are prepared by dissolving pure calcium carbonate. CaCO3, in dilute hydrochloric acid. A 1.745-g sample of CaCO3 is placed in a 250.O-mL volumetric flask and dissolved in HCI. Then the solution is diluted to the calibration mark of the volumetric flask. Calculate the resulting molarity of calcium ion.arrow_forward4.59 Aluminum dissolves in HCI according to the equation written below, whereas copper does not react with HCl. 2Al( s )+6HCl( aq ) 2AlCl 3 ( aq )+ 3H 2 ( g ) A 35.0-g sample of a copper—aluminum alloy is dropped into 750 mL of 3.00 M HCl, and the reaction above proceeds as far as possible. If the ahoy contains 77.1% Al by mass, what mass of hydrogen gas would be produced?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781285199023Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781285199023
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning