
Chemistry & Chemical Reactivity
9th Edition
ISBN: 9781133949640
Author: John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What is TRUE about the relationship between temperature and
volume?
Question 31 options:
|
|
temperature and volume are inversely related |
|
|
temperature and volume are directly related |
|
|
temperature and volume have no mathematical relationship |
|
|
temperature and volume are exponentially related |
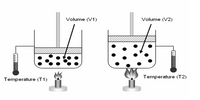
Transcribed Image Text:Volume (V1)
Volume (V2)
Temperature (T2)
Temperature (T1)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In the combustion of ethanol, 20.50 g of carbon dioxide gas was produced, What is the volume in mL of ethanol was used? Density of ethanol is 0.789 g/mL. C2H5OH (1) + 302 (g) → 2 CO2 (g) + 3 H2O (1)arrow_forward9.81m/s^2 to ft/s^2arrow_forwardConvert the (US) price of gas of $3.56/gallon into (British) E/L. Hints: 1 £ = $1.35; 1 gallon = 3.785 L. Do not include units in your submitted answer.arrow_forward
- A typical atmospheric pressure in Leadville, Colorado (elevation 10,200 feet) is 68 kPa. Use the graph above to determine the boiling point of water at this elevation. °Carrow_forwardBelow are 3 real life situations. Pick two (2) them and explain ALL the science involved in the scenario. This means describe things like what is happening to the gas/liquid/solid molecules or atoms, which principles or laws are at work, why what is happening is happening, and how you know. At sea level, a pot of water will boil at 100 °C. Suppose you wanted to boil the same amount of water high in the Rocky Mountains. What do you expect the temperature will need to be (generally speaking)? Why? A can of soda sits in a sunny spot on the window sill for several hours. It is then opened at the same time as a cold can of soda from the refrigerator. What happens and why? Weather balloons do not float out into space. They reach an altitude of about 15 miles above the surface (3x the height of Mt Everest) and they burst. Why?arrow_forwardI am getting hung up on the X 10^-2. Am I supose to do 10x10 there?arrow_forward
- The liquid 3-chloro-1,1,1-trifluoropropane has a density of 1.33 g/mL at 20 °C. If a 2.12 kilogram sample of this compound is needed, how many liters of the liquid at 20 °C must be provided?arrow_forwardA chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of carbon tetrachloride, diethylamine, methyl acetate, tetrahydrofuran, and acetone. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information:arrow_forwardA chemist working as a safety inspector finds an unmarked bottle in a lab cabinet. A note on the door of the cabinet says the cabinet is used to store bottles of methyl acetate, chloroform, ethanolamine, diethylamine, and tetrahydrofuran. The chemist plans to try to identify the unknown liquid by measuring the density and comparing to known densities. First, from his collection of Material Safety Data Sheets (MSDS), the chemist finds the following information: liquid methyl acetate chloroform ethanolamine diethylamine tetrahydrofuran density 0.93 g ml 1.5 g ml 1 -1 1.0 g ml 1 0.71 g ml 0.89 g ml. Next, the chemist measures the volume of the unknown liquid as 1005. cm' and the mass of the unknown liquid as 1.49 kg. Calculate the density of the liquid. Be sure your answer has the correct number of significant digits. Given the data above, is it possible to Identify the liquid? If it is possible to identify the liquid, do so. 0 gml. yes no methyl acetate chloroform ethanolamine…arrow_forward
- A river pollutant is found to have a concentration of 0.0031 % m/v. If the density of the river water is 1.183 g/ml, what is the concentration of the pollutant in ppm? Proper rounding of your answer is required.arrow_forwardA pressure versus volume (pV) diagram for a system is shown in the figure. The arrows of the curve indicate the 1 direction of the process, and the points of interest are labeled. The values for the points in the diagram are shown in the table. Volume (m³) Pressure (Pa) 2 3 Vo = 25.8 Po = 1.00 × 104 V = 20.5 P1 = 1.00 × 104 V2 = 17.4 P2 = 5.13 × 103 V3 = 13.9 P3 = 5.13 × 103 V4 = 13.9 P4 = 3.20 × 10³ Vs = 7.00 P5 = 1.19 × 10³ Volume (m³) Calculate the amount of work done on the system from 0-2 Wo2) and then for the entire curve from 0-5 (Wos ). Wo2 = J Vị = 20.5 Pi = 1.00 × 104 V2 = 17.4 P2 = 5.13 × 103 V3 = 13.9 P3 = 5.13 × 103 V4 = 13.9 P4 = 3.20 × 103 Vs = 7.00 P5 = 1.19 × 10³ Volume (m³) Calculate the amount of work done on the system from 0–2 Wo2) and then for the entire curve from 0-5 (Wos ). Wo2 = J Wos = J Pres: Pressure (Pa)arrow_forwardA chemist prepares a solution of iron(II) bromide (FeBr₂) by measuring out 491. g of iron(II) bromide into a 500. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's iron (II) bromide solution. Be sure your answer has the correct number of significant digits. ? mol/L x10 X Ś 00. 18 Ararrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:OpenStax

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning