
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
thumb_up100%
I am getting hung up on the
X 10^-2. Am I supose to do 10x10 there?
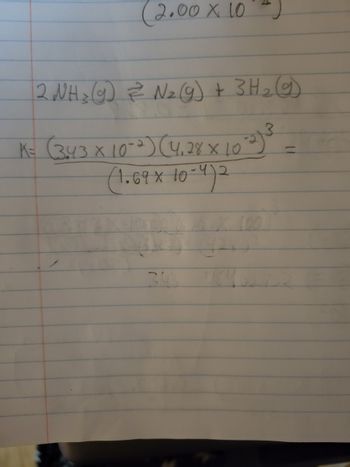
Transcribed Image Text:(2.00 x 10¹2)
2 NH ₂ (g) 2² N₂ (g) + 3H₂(g)
K = (3.43 × 10-²) (4.28 × 10-²3) ³
(1.69 × 10-4)2
343
Expert Solution
arrow_forward
Step 1
Since,
Equilibrium constant or Kc is the ratio of the equilibrium concentrations of product over equilibrium concentrations of reactants all raised to the power of their stoichiometric coefficients.
Thus,
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If the specific gravity of a liquid mercury is 13.6, calculate the mass in pounds of 2500 mL of that liquid. Use proper units and sig figs. I stumble upon this question and I am struggling to answer it. I was curious on how to go about solving this question.arrow_forwardThe engine in a boat has a displacement of 350 inches^3 What is the displacement of this engine in ft^3? In cm^3? In liters? Show steps and correctly round answersarrow_forwardPlease help me find volume 285m^3 in each of these unit's with work! Thanks so mucharrow_forward
- 2- Suppose that a liquid is 11 times denser than water. If you were to sip this liquid on the top of Mount Everest using a straw, what is the maximum Length in meter your straw would be? (dwater:0.997 g/mL; g:9.773m/s^2)arrow_forwardJenny Craig promised a 400 lb’s lady to lose an average of 1.10 lb weight every day. How much will she weigh at the end of the first year (365 days), in kilogram, kg (1kg = 2.2 lb)? Will she still be alive?arrow_forwardHow do I set up this conversion?arrow_forward
- The specific gravity of a patient’s urine sample was measured to be 1.008. Given that the density of water is 1.000g/mL at 48C, what is the density of the urine sample?arrow_forwardConvert 26.31 mg into amu, using dimensional analysis.arrow_forwardI need the inverse of each volumes Volume 1: 10.0 nm Volume 2: 6.0 nm Volume 3: 14.4 nm Volume 4: 11.3 nm Volume 5: 15.0 nmarrow_forward
- Using dimensional analysis convert 6.5in^2/sec to min^2/sec . Show your work step by step.arrow_forwardIf you want to make 1 L of 1X of TGS using 2 x TGS stock, how much of the 2 x do you need? Report your answer in units of L, with 1 decimal place.arrow_forwardWhat is the mass of (1.000) Litre of gold? The density of gold is 19.3 grams/cm^3arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY