Question
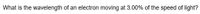
Transcribed Image Text:What is the wavelength of an electron moving at 3.00% of the speed of light?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Calculate the de Broglie wavelength of a proton moving at 2.60 x 104 m/s and 1.99 x 108 m/s. (a) 2.60 x 104 m/s m (b) 1.99 x 108 m/sarrow_forwardA photon has momentum of magnitude 2.48 x 10-28 kg.m/s. (a) What is the kinetic energy of this photon? (b) What is the wavelength of this photon? (c) In what region of the electromagnetic spectrum does it lie?arrow_forwardWhat is the wavelength of an electron moving at 1.5 %of the speed of light? Use nonrelativistic calculations.arrow_forward
- What is the wavelength of a photon with frequency f= 5x10^14 Hz??arrow_forwardWhat is the energy of a photon if it's frequency is 1.00s-1?arrow_forwardBy Thomson's time, it was known that excited atoms emit light waves of only certain frequencies. In his model, the frequency of emitted light is the same as the oscillation frequency of the electron or electrons in the atom. What would the radius of a Thomson-model atom have to be for it to produce red light of frequency 4.55×1014 HzHz ? (see Appendix FF from the textbook for data about the electron)arrow_forward
arrow_back_ios
arrow_forward_ios