
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
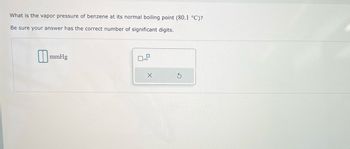
Transcribed Image Text:What is the vapor pressure of benzene at its normal boiling point (80.1 °C)?
Be sure your answer has the correct number of significant digits.
mmHg
x10
X
Expert Solution
arrow_forward
Step 1 Introduction
The vapor pressure of a substance is the pressure exerted by its vapor when it is in equilibrium with its liquid or solid form at a given temperature.
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A. an experiment was done to determine the value of the ideal gas constant( R). hydrogen gas was generated and collected at a given temperature and pressure 1.44X 10 ^-3 moles of hydrogen gas occupied 35.9 mL at 758.0 MMHG and 23.2 Celsius based on these values, calculate the experimentally determine value of R (L x atm/mol x K). B. Using the ungrounded number for the previous problem determine the percent error for the experimentally determined value of R.arrow_forwardFor many purposes we can treat ammonia (NH3) a as an ideal gas at temperatures above its boiling point of -33. °C. 3 Suppose the pressure on a 1.0 m sample of ammonia gas at -2.00°C is reduced to one-third its initial value. Is it possible to change the temperature of the ammonia at the same time such that the volume of the gas doesn't change? If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C. yes no °C x10 Sarrow_forwardFor many purposes we can treat propane (C₂Hg) as an ideal gas at temperatures above its boiling point of -42. °C. Suppose the temperature of a sample of propane gas is lowered from 21.0 °C to -24.0 °C, and at the same time the pressure is changed. If the initial pressure was 0.34 kPa and the volume decreased by 55.0%, what is the final pressure? Round your answer to the correct number of significant digits. kPa X Śarrow_forward
- Oo.140. Subject:- Chemistryarrow_forwardFor many purposes we can treat butane (C,H10) : as an ideal gas at temperatures above its boiling point of – 1. °C. Suppose the pressure on a 43.0 g sample of butane gas at 14.0°C is reduced to one-third its initial value. Oyes Is it possible to change the temperature of the butane at the same time such that the volume of the gas doesn't change? к10 no If you answered yes, calculate the new temperature of the gas. Round your answer to the nearest °C.arrow_forwardWhen collecting a gas over water it is necessary to correct for the vapor pressure of water in order to determine the pressure of the gas you are collecting. We will do this by using a graph of ln P in mmHg versus 1/T where T is the temperature in Kelvin. The equation is y = -5206.4 x + 20.621 (With an R^2 = 0.9999). If you collect a gas at 26.8 ℃, what is the value of “x” that you want to use in the equation. (Keep the answer for the next question). (Keep extra digits, you do not want to round in the middle of this calculation). Select one: a. 0.003660992 b. 0.037313433 c. 0.003378378 d. 299.95 e. 0.0273150 f. 273.15 g. None of these h. 0.003333889 i. 0.0027315arrow_forward
- When limestone (solid CaCO3) is heated, it decomposes into lime (solid CaO) and carbon dioxide gas. This is an extremely useful industrial process of great antiquity, because powdered lime mixed with water is the basis for mortar and concrete - the lime absorbs CO₂ from the air and turns back into hard, durable limestone. Suppose some calcium carbonate is sealed into a limekiln of volume 550. L and heated to 520.0 °C. When the amount of CaCO3 has stopped changing, it is found that 8.46 kg have disappeared. Calculate the pressure equilibrium constant K, this experiment suggests for the equilibrium between CaCO3 and CaO at 520.0 °C. Round your answer to 2 significant digits. P Note for advanced students: it's possible there was some error in this experiment, and the value it suggests for K does not match the accepted value. 0 Xarrow_forwardFor many purposes we can treat ammonia (NH,) as an ideal gas at temperatures above its boiling point of –33. °C. Suppose the temperature of a sample of ammonia gas is raised from – 25.0 °C to 17.0 °C, and at the same time the pressure is changed. If the initial pressure was 0.15 kPa and the volume decreased by 50.0%, what is the final pressure? Round your answer to 2 significant digits. kPa 1 Don't Know Submit etv DII esc %23 24 6 7 09 2 4 OP E T Y.arrow_forwardCalculate the total pressure (in atm) of a mixture of 0.0200 mol of helium, He, and 0.0100 mol of hydrogen, H2, in a 0.50 L flask at 10°C. Assume ideal gas behavior. Given that R = 0.0821 L'atm/(mol·K). a. 0.279 atm. b. 0.465 atm. c. 0.349 atm. d. 0.697 atm. O e. 1.39 atm.arrow_forward
- Use your text or other reference to find the partial pressure of water at 25°C, 26°C, and 27°C and record those values.arrow_forwardGaseous CO exerts pressure of 45.6 mmHg in a 56.0 L tank at 22°C was released to a room with a volume of 2.70 x 104L. The room has another two gaseous that are 5.50 g of NO2 and 8.034 x 1022 atoms of SO2 at 22°C. Calculate the partial pressure of CO in the room at 22°C. •Ans : P = 0.09458 mmHg Calculate the total pressure in the room and the molarity of CO gas in the room. •Ans: 5.14 x 10-6 M, Total pressure 2,7 x 10-4 atm Calculate the density of the mixture in the room and the mass percentage of SO2 gas in the room. 6.00027arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY