
Biochemistry
9th Edition
ISBN: 9781319114671
Author: Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Question
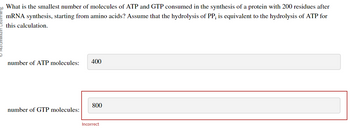
Transcribed Image Text:What is the smallest number of molecules of ATP and GTP consumed in the synthesis of a protein with 200 residues after
mRNA synthesis, starting from amino acids? Assume that the hydrolysis of PP; is equivalent to the hydrolysis of ATP for
this calculation.
number of ATP molecules:
number of GTP molecules:
400
800
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- Consider the graphic representation shown below for the free energy change for the overall reaction of Fructose-6-phosphate and ATP to form the products Fructose-1,6-biphosphate and ADP in the presence (blue line) or absence (red line) of enzyme. Match each of the arrows (labeled A, B, C, or D) indicated in the graph with an apropriate description by dragging the letters into their corresponding box. C D reactants Fructose-6-phosphate + ATP A products Fructose-1,6-biphosphate + ADP Progress of the Reaction Reset Help D BAc В C Energy of activation for the forward reaction in the absence of enzyme Energy of activation for the forward reaction Energy of activation for the reverse reaction in absence AG for the reaction the presence of enzyme of enzyme Energyarrow_forwardWhich of the following five processes are points of regulation for controlling enzyme-mediated reactions? Select 5 correct answer(s) Dihydrolipidation of arginine Disulfide bond acetylation Protein targeting RNA processing Cytosine methylation Proteolytic processing Protein degradationarrow_forwardIn trimetric G proteins the only function of the bamma y complex is to allow reactivating of the a subunit ? True or falsearrow_forward
- Consider an enzyme that catalyzes the reaction S2 P, by the following simple reaction mechanism: k, E + S 2 E•S →E kcat + P Suppose the enzyme acquires a mutation that causes k1 to be 10-times smaller than for the wild-type (non-mutant) enzyme. Suppose you measure the initial reaction rate (vo) at several different [S] for the mutant and the wild-type enzymes. Under what conditions would the mutation have a greater effect on the reaction rate (vo) of the mutant enzyme compared to the wild-type enzyme - at very low [S], or at very high [S]? Explain briefly how you decided.arrow_forwardPlease asnwer the following question: Suppose you specifically want to measure the amount of beta-glucose present in a sample. You have an enzyme that reacts specifically with the beta form of the sugar,and produces a signal that is easy to measure. However, every time you try to measure the amount of beta-glucose, mutarotation occurs and the alpha form of the sugar is converted into beta!! Can you suggest a simple way of overcoming this problem so you could measure only the beta form of the sugar? HINT: Is there a way you can prevent mutarotation?arrow_forwardMany enzymes are switched "on" by attachment of a phosphate group at a specific serine somewhere on the protein (phosphorylation). The basic reaction is: E + ATP2 Ep + ADP Po SERINE PHOSPHO SERINC (Note the "squiggles" before the backone amide and carbonyl indicate the polypeptide chain continues on either side of the serine). For phosphorylation to have this effect, there has to be some equilibrium between inactive and active forms conformations of the enzyme: [Eactive] [Einactive] Einactive 2 Eactive; K* The same basic equilibrium must exist for the phosphorylated protein: [Ep,active] [Ep,inactive] EP,inactive 2 Ep,active; Kp = (a) If phosphorylation increases the measured activity of the enzyme, is K* or K larger? Why? (b) Does the phosphorylation site need to be near the site where the enzyme binds its substrate (e.g. the reactant whose chemistry it catalyzes)? Why or why not?arrow_forward
- Mutations in adenylate kinase have led to a hyperactive enzyme that ultimately ends up elevating ADP levels in a cell.Calculate the EC (energy charge) given the following atypical adenylate concentrations for the cell containing the mutant adenylate kinase: ATP = 0.5 ??��, ADP = 12.2 ??��, AMP = 80 ??��.arrow_forwardInsulin resistance, as occurs in type 2 diabetes, may lead to increased ketone production and release into blood. Describe the biochemistry that links insulin resistance and ketone production. Compare the cellular energy (e.g. ATP) required and produced when glycogen is synthesize and hydrolyzed, respectively. Compare and contrast the mechanism of fatty acid synthase with translation. When young rats are placed on a totally fat acid free diet, they grow poorly, develop a scaly dermatitis, lose hair, and soon die these symptoms that can be prevented if plant material is included in the diet. Why?arrow_forwardThe activity of the enzyme β-galactosidase produced bywild-type cells grown in media supplemented with different carbon sources is measured. In relative units, thefollowing levels of activity are found:Glucose Lactose Lactose + glucose0 100 1Predict the relative levels of β-galactosidase activity incells grown under similar conditions when the cells arelacI−, lacIS, lacO+, and crp−.arrow_forward
- From the complete oxidation of glucose (glucose → 6CO2), how many total nucleotide triphosphates are yielded (be sure to deduct payback) as part of substrate level phosphorylation?arrow_forwardWhich of the following would result in an increase in the amount of F-2,6-BP? (select all that apply) Group of answer choices high blood glucose activation of fructose bisphosphatase 2 increased phosphofructokinase 2 activity increased glucagon secretion increased phosphorylation of the bifunctional enzymearrow_forwardFor the following problem, indicate whether enzyme is repressed (-) or produced (+). Please show all possible outcomes.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON