
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
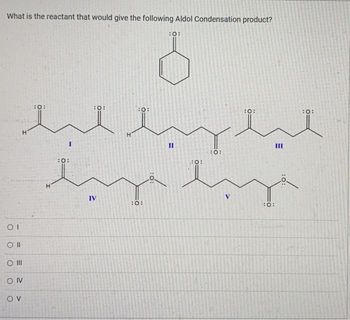
Transcribed Image Text:What is the reactant that would give the following Aldol Condensation product?
01
0 |
0 |||
O IV
OV
H
H
تبار نہید لبد
بد مبد
IV
:O:
ة
:0:
:0:
:O:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Please draw an arrow pushing mechanism of this reactionarrow_forwardWhich arrow-pushing patterns are employed in the following mechanism? :ӧсн, Hас — Br Proton transfer; rearrangement. Nucleophilic attack; loss of leaving group. Loss of leaving group; rearrangement. Nucleophilic attack; proton transfer.arrow_forwardWhat type of mechanistic step is illustrated below? proton transfer loss of a leaving group nucleophilic attack rearrangementarrow_forward
- Curved arrows are used to illustrate the flow of electrons. Follow the arrows and draw the intermediate and product in this reaction. Include all lone pairs. Ignore stereochemistry. Ignore inorganic byproducts. :O: H :OCH3 H OH CH3 CH3OH2+ protonation CH3OH deprotonation H3C CH3OH ОН nucleophilic addition -H Draw Intermediate Qarrow_forwardPredict the most likely product of the following reaction and draw the step-wise mechanism. „NAOHarrow_forwardtaneon lo robio i guiwollol o n 6A. Draw all of the possible aldol addition and condensation products from the following reaction (you may ignore stereochemistry of aldol products but not condensation): NaOH OMe ? H3C CH3 H OMe Jobong oryloby vithob plamarrow_forward
- Consider the mechanism for the given nucleophilic substitution reaction. Part:0/5 Part 1 of 5 と Predict the nucleophilic substitution product. 2CH3CH2OHarrow_forward10arrow_forward10:47 AM Sun Nov 27 ← Question 28 of 41 Draw the Alkyl Halide Rea nt @72% Draw an alkyl halide that produces ONLY the following alkene in an E2 elimination. Ignore any inorganic byproducts. Strong Base Submitarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY