
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
What is the rate expression for the overall reactio based on the data?
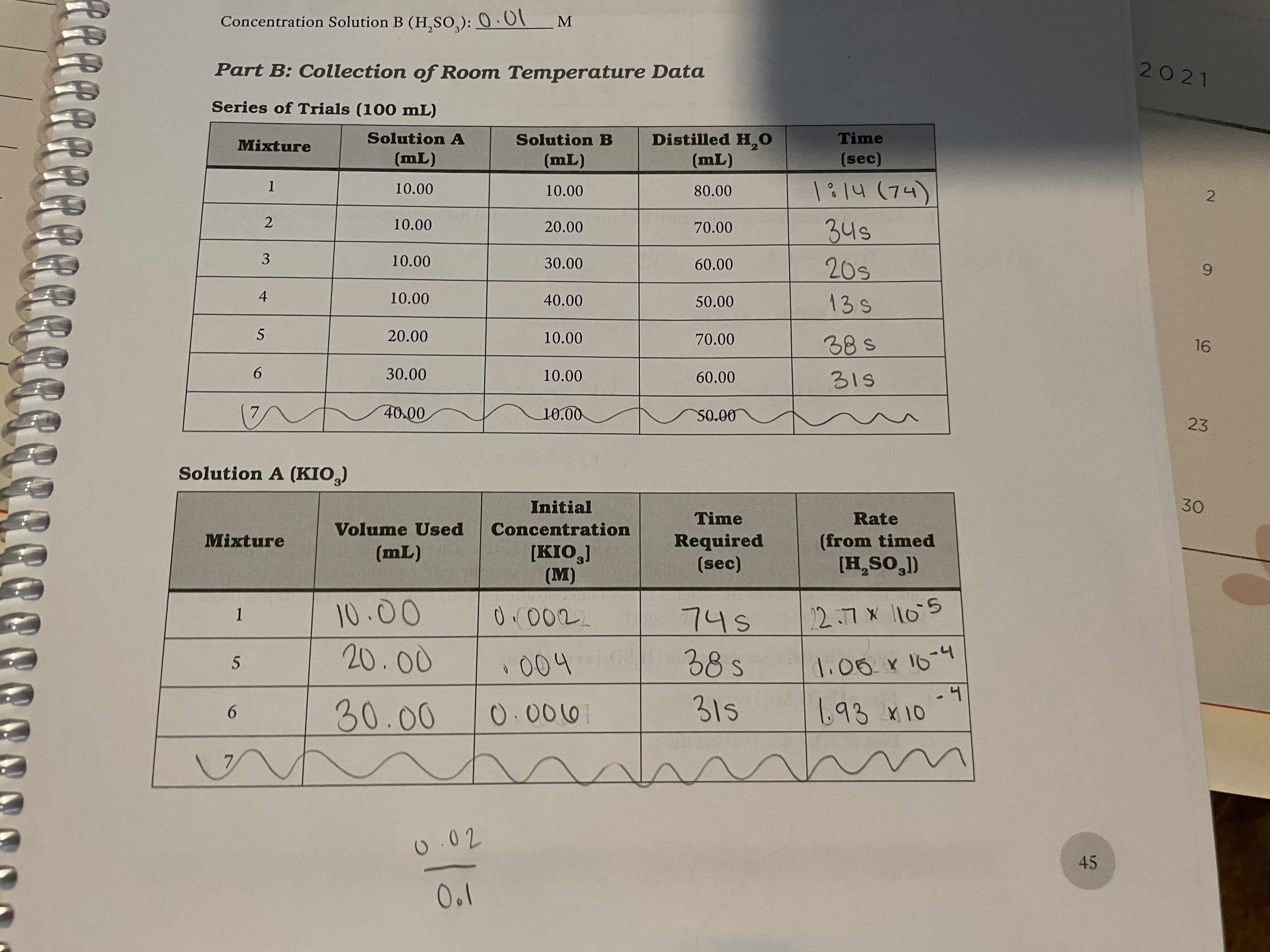
![Initial
Time
Volume
Rate
1/[H,SO,]
(M')
Required
(sec)
Concentration
Mixture
Used
In[H,SO,]
(M/s)
[H,SO,]
(М)
(mL)
10
1000
7니
0.0135
1
| x 10
--9,21
20
2 x 10-4-8.51.500
34
2
O. 0147
30
3 x 10
333
20
3
8.11
O.016
40
X lo4-7. 82
250
13
6.0192
4
Part C: Temperature Effects on Reaction Rates
Temp °C
Time Required (sec)
20
20s
Mixture 3 (r.t.)
Trial 1 (cold)
5
23s
Trial 2 (warm)
33.7
19s](https://content.bartleby.com/qna-images/question/abc97ca2-5307-4b01-b84c-b32d2ca85d88/28f26cf9-4afa-410b-9f97-5be03a94828d/xefxiar_thumbnail.jpeg)
Transcribed Image Text:Initial
Time
Volume
Rate
1/[H,SO,]
(M')
Required
(sec)
Concentration
Mixture
Used
In[H,SO,]
(M/s)
[H,SO,]
(М)
(mL)
10
1000
7니
0.0135
1
| x 10
--9,21
20
2 x 10-4-8.51.500
34
2
O. 0147
30
3 x 10
333
20
3
8.11
O.016
40
X lo4-7. 82
250
13
6.0192
4
Part C: Temperature Effects on Reaction Rates
Temp °C
Time Required (sec)
20
20s
Mixture 3 (r.t.)
Trial 1 (cold)
5
23s
Trial 2 (warm)
33.7
19s
Expert Solution
arrow_forward
Step 1
The rate expression for the reaction can be written as:
where,
x = Order w.r.t KIO3
y = Order w.r.t H2SO3
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- At a certain temperature the rate of this reaction is first order in with a rate constant of Suppose a vessel contains at a concentration of . Calculate the concentration of in the vessel seconds later. You may assume no other reaction is important. Round your answer to significant digits.arrow_forwardAs stated in Arhenius equation, rate of reaction increases as pre exponential factor.....temperature....and activation energy doesnt change . Is the sentence above correct? Explain whyarrow_forward1) The reaction order with regards to nitrogen monoxide gas is : The reaction order with regards to hydrogen gas is : 2) The student performs Trial 4 with the same initial concentrations as Trial 1 but a higher temperature. The initial rate of Trial 4 should be : a) equal to b) higher than c) lower than Trial 1. The student forms performs Trial 5 with initial concentrations of both reactants of 0.20M. The initial rate of Trial 5 should be a) equal to b) higher than c) lower than Trial 1arrow_forward
- At what point on a Potential Energy curve would you find the energy needed for an effective collision?arrow_forwardFor a reaction which is first order with respect to one reactant, plotting result in a straight line. versus time will one over reactant concentration O reactant concentration O None of the other four answers is correct. O In of reactant concentration one over In of reactant concentrationarrow_forwardWhat are the units of the rate constant for a third order reaction? Can you explain with equations..?arrow_forward
- After 20 min, a reactant has decomposed to 85% of its original concentration. Which order would the reaction need to be to allow you to solve for the rate constant, ?, with only this information?arrow_forwardHow does the increase in the concentration of one reactant affect the rate of reaction if all other terms are unchanged? (Please show all information this question)arrow_forwardMatch the quantities that yield straight lines for the given types of reactions molarity vs. time zero order rate equations inverse molarity vs. time secondorder rate equations In molarity vs. time firstorder rate equations inverse molarity vs. inverse time not a straight line for any type & へ へarrow_forward
- In Chemical Kinetics, the unit in which rate is measured is independent of the order of the reaction. Correct?arrow_forwardIn an experiment to determine the rate law for the decomposition of acetaldehyde, after collecting her data, a student plot three graphs(see attached image): [acetaldehyde] vs. time, In [acetaldehyde] vs. time and 1/[acetaldehyde] vs. time. Her graphs at 427°C are: 1. Based on these, what is the order of the reaction with respect to [acetaldehyde]? Briefly explain.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY