
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
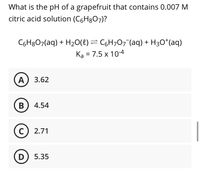
Transcribed Image Text:What is the pH of a grapefruit that contains 0.007 M
citric acid solution (C6H8O7)?
C6H8O7(aq) + H20(8) = C6H707¯(aq) + H3O*(aq)
Ka = 7.5 x 10-4
A) 3.62
B) 4.54
C
2.71
D) 5.35
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Similar questions
- Use this balanced equation to solve the following problem: NazCO3 (5) + 2 HCI (ag) - H20 () + CO2 (g) + 2 NaCl (ag) 6.91 grams of sodium carbonate are added to 0.053 moles of hydrochloric acid. How many grams of carbon dioxide will be produced? What is the limiting reactant? (you should select 2 answers) O hydrochloric acid is limiting O sodium carbonate is limiting both reactants are limiting O there is no limiting reactant O 1.17 g carbon dioxide O 2.33 g carbon dioxide O 2.87 g carbon dioxide O 5.74 g carbon dioxide O 1.01 g carbon dioxide O 0.37 g carbon dioxide O 0.41 g carbon dioxidearrow_forward1arrow_forwardA student has prepared a solution containing two acids : H2A ( PK , = 2.1 , 7.2 ) and H2B ( pKg = 6.4 , 10.3 ) . If the solution is at a pH of 8.05 , which combination shown below is buffering the solution ?arrow_forward
- How many grams of antifreeze would have to be added to 15 kg of water in the trucks cooling system to keep it from freezing during a cold Ohio winter temperatures of -20° Carrow_forward1. Water Quality Analysis - A public utility announces the monthly average concentrations of several ionic species in the drinking water. The list is given below (atomic weights are given in parentheses): Calcium (40) = 8.6 mg/L Magnesium (24)=0.77 mg/L Potassium (39) = 0.64 mg/L Sodium (23) = 3.7 mg/L a) What is the ratio of [HCO3]/[CO3²] in this water Chloride (35.5) -3.0 mg/L Fluoride (19)- 0.84 mg/L Sulfate (S-32; O=16)= 3.4 mg/L pH=8.46arrow_forwardPlease correct answer and don't use hand ratingarrow_forward
- 1. On the following page, construct a graph of n'H, (moles hydrogen per gram of sample) vs. % Al. Use Equation (12): (100-%A¹(0.0153) (A)(0.0556)+( H₂ Simply solve this equation for n'H, using % Al = 0 and % Al = 100, then draw a straight line through these points. n H₂ = % (12)arrow_forwardQ22. A solution is prepared by mixing 25.0 g of naphthalene (C₁0Hs) with 0.750 L of liquid CS2 (density = 1.263 g/mL). What is the concentration of naphthalene in parts per million (ppm)? a) 7.34×10¹ ppm d) 2.96x10¹ ppm b) 5.01x10¹ ppm e) 4.62×10¹ ppm c) 2.57x10¹ ppm Q23. A 10.00 g of an organic compound is dissolved in 50.00 g of benzene (k = 6.940 °C/m). The freezing point of the solution is 1.280 °C below that of pure benzene. What is the molar mass of the compound? a) 1084 g/mol d) 3253 g/mol b) 2169 g/mol e) 776.5 g/mol c) 1627 g/molarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The