
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN: 9781259696527
Author: J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Question
Is it 25oC ? Not sure
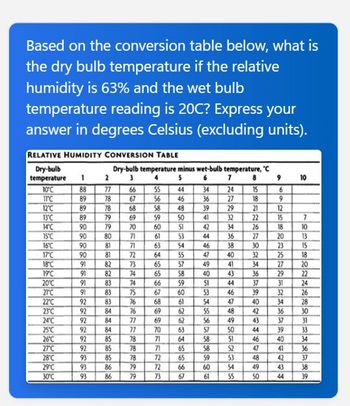
Transcribed Image Text:Based on the conversion table below, what is
the dry bulb temperature if the relative
humidity is 63% and the wet bulb
temperature reading is 20C? Express your
answer in degrees Celsius (excluding units).
RELATIVE HUMIDITY CONVERSION TABLE
Dry-bulb
temperature 1
10°C
11°C
12°C
13°C
14°C
15°C
16°C
17°C
18°C
19°C
20°C
21°C
22°C
23°C
24°C
25°C
26°C
27°C
28°C
29°C
30°C
ஐ ஐ ஐ ஐ 8 8 8 8 ச ச ச க & C B B B 6 சி 8 8
88
89
89
90
91
91
91
89 79
91
92
~ES
92
2
92
77
92
78
78
22285 0
93
90 80 71
79
81
81
82
83
Dry-bulb temperature minus wet-bulb temperature, "C
4 5
55 44
3
6
8
34
56 46 36
85382EFEERKREFERPRER
***833333333$$RRERES
5538388
66
84
67
84
68
69
70
92 84 76
86
71
72
83 75
82 73 65
74
92 83 76 68
77
77
78
93 85 78
78
93 86 79
58 48 39
41
59
60
79
61
74 66 59
63
64
65
67
69
69
70
71
71
72
72
50
51
53
73
54
55
57
IEEE 85%
61
62
63
285XXXSXS48558RAA
64
65
65
44
66
67
36 27
38
40
41
43
44
46 39
47
48
62 56 49
46
47
60 53
49
40
51
57
59
60
7
24
27
61
29
32
34
50
85 3 3 3 3
51
52
53
54
RESEARCHER
55
15
18
21
22
26
30
32
34
36
37
42
43
44
46
47
48
49
9
50
6
CAFELEKRAAGGG
12
15
40 34
18
20
23
25
27
29
32
36
39
40
42
43
44
10
7835R2228825318ARS
10
20
24
26
30
34
36
39
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Similar questions
- A manufacturer advertises that a particular set of safety goggles has a optical density of at least 1.8. If the incident ultraviolet radiation for a particular process is 230 mW/cm2; how much energy in mW/cm2 will reach the eye if these glasses are worn?arrow_forwardConvert 23lbf ∙ ft/min2 to its equivalent in kg ∙ cm/s2. NOTE: it is lbf not lb.arrow_forwardCalculate the expected yield of lead if 71.0 kg of lead oxide is heated with 71.0 kg of carbonarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The

Introduction to Chemical Engineering Thermodynami...
Chemical Engineering
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind...
Chemical Engineering
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY

Elements of Chemical Reaction Engineering (5th Ed...
Chemical Engineering
ISBN:9780133887518
Author:H. Scott Fogler
Publisher:Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:9781285061238
Author:Lokensgard, Erik
Publisher:Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:9780072848236
Author:Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:McGraw-Hill Companies, The