
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
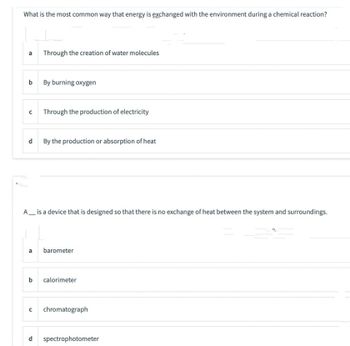
Transcribed Image Text:What is the most common way that energy is exchanged with the environment during a chemical reaction?
a
b
Through the creation of water molecules
By burning oxygen
с Through the production of electricity
d By the production or absorption of heat
A__ is a device that is designed so that there is no exchange of heat between the system and surroundings.
a barometer
b calorimeter
с chromatograph
d spectrophotometer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider these reactions: Reaction 1: H₂(g) + Cl₂(g) → 2HCl(g) 2HCl(g) AH = -184.6 kJ Reaction 2: 20F2(g) → O₂(g) + 2 F₂ (g) AH = −49.4 kJ Reaction 3: N₂(g) + 2O₂(g) →→→ 2NO₂(g) AH = +66.4 kJ Use Reaction 2. How much energy (in kJ) is released when 69.0 g of oxygen difluoride decompos Answer: kJ (enter a positive value)arrow_forwardAn isolated system is one that O exchanges heat and matter with the surrounds. exchanges neither heat nor matter with the surroundings. O exchanges matter but not heat with the surroundings. O exchanges heat but not matter with the surroundings.arrow_forwardHydrogen sulfide, H2 S, is produced during decomposition of organic matter. When 0.4300 mol H2S burns to produce SO2 (g) and H20(g), -222.7 kJ of heat is released. What is this heat in kilocalories? Heat kcalarrow_forward
- Reactions are classified as either exothermic or endothermic. Exothermic reactions feel hot (e.g., a burning campfire), whereas endothermic reactions feel cool (e.g. squeezing an instant cold pack). A thermometer measures the temperature of the surroundings in a calorimeter to determine the amount of energy being transferred by the system. Water molecules speed up when they gain heat and slow down when they lose heat; note that you can track both the cold and hot water molecules in the simulation based on the shade of red of the larger central oxygen atom by checking the box labeled Show microscopic view. Select the Experiment tab in the simulation, and then click Run Demonstration. When observing the simulation, pay particular attention to the temperature change and movement of the molecules by clicking Show microscopic view when HCl and NaOH neutralize each other in the fourth experiment. Use your observations to complete the following sentences. Match the words in the left column to…arrow_forwardTitanium reacts with iodine to form titanium(III) iodide, emitting heat, via the following reaction: 2Ti(s)+3I2(g)→2TiI3(s), ΔHrxn=−839kJ Determine the mass of titanium that reacts if 1.53×103 kJ of heat is emitted by the reaction. Express your answer to three significant figures and include the appropriate units. Determine the mass of iodine that reacts if 1.53×103 kJ of heat is emitted by the reaction. Express your answer to three significant figures and include the appropriate units.arrow_forward[References] Use the References to access important values if needed for this question. When HBr(g) reacts with Cl₂(g) according to the following reaction, 9.902 kcal of energy are evolved for each mole of HBr(g) that reacts. Complete the following thermochemical equation. 2 HBr(g) + Cl₂(g) → 2 HCI(g) + Br₂(g) AH = kcalarrow_forward
- Expressing amounts of energy in different energy units is necessary to solve many chemistry problems. For practice, complete the following table. The Joule (J) is the SI unit of energy. 1 calorie (cal) = 4.184 J; 1 kcal = 1000 cal J kJ kcal 549 0.684 0.226arrow_forwardConsider these reactions, where M represents a generic metal. 1. 2 M(s) + 6 HCI(aq) 2 MCI, (аq) + 3 Н, (2) AH1 = -556.0 kJ 2. HCl(g) HCl(aq) AĦ2 = -74.8 kJ 3. H, (g) + Cl, (g) → 2 HCI(g) ΔΗ = -1845.0 kJ 4. MCI, (s) – MCI, (aq) AĦ4 = -493.0 kJ Use the given information to determine the enthalpy of the reaction 2 M(s) + 3 Cl, (g) – - 2 MCl, (s) -556.0 ΔΗ- Incorrectarrow_forwardExplain what the equation Q = m•C•ΔT means and how it relates to energy and calorimetry.arrow_forward
- A chemical reaction takes place inside a flask submerged in a water bath. The water bath contains 2.70kg of water at 39.2°C . During the reaction 128.kJ of heat flows out of the bath and into the flask. Calculate the new temperature of the water bath. You can assume the specific heat capacity of water under these conditions is 4.18·J·g−1K−1 . Be sure your answer has the correct number of significant digits.arrow_forwardWhen 145 mL of 0.212 M NaCl(aq) and 145 mL of 0.212 M AgNO3(aq), both at 21.1°C, are mixed in a coffee cup calorimeter, the temperature of the mixture increases to 23.7°C as solid AgCl forms. NaCl(aq) + AgNO3(aq) → AgCl(s) + NaNO3(aq) This precipitation reaction produces 3.14 ✕ 103 J of heat, assuming no heat is absorbed by the calorimeter, no heat is exchanged between the calorimeter and its surroundings, and that the specific heat and density of the solutions are the same as those for water (4.18 J/g·°C, and 0.997 g/mL, respectively). Using this data, calculate ΔH in kJ/mol of AgNO3(aq) for the given reaction.arrow_forward6. An enthalpy change is a. the difference in the kinetic energy of the reactants and the products in a chemical change b. the difference in the potential energy of the reactants and the products in a chemical change c. the difference in enthalpies of the reactants and the products in a chemical change d. the sum of the potential and kinetic energies of the products e. the sum of the potential and kinetic energies of the reactants 7. Which statement concerning the Law of Conservation of Energy is not true? a. it applies to all chemical changes b. it involves all different forms of energy c. it applies to nuclear reactions d. it includes potential energy e. it involves heat content of substancesarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY