
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
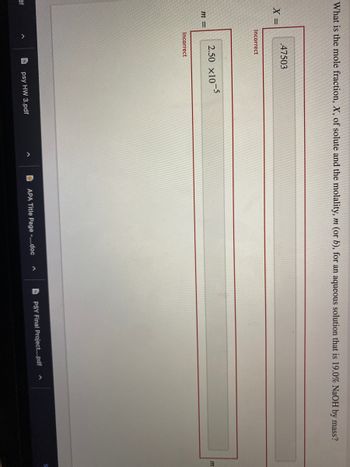
Transcribed Image Text:What is the mole fraction, X, of solute and the molality, m (or b), for an aqueous solution that is 19.0% NaOH by mass?
df
X =
m=
.47503
Incorrect
2.50 x10-5
Incorrect
psy HW 3.pdf
APA Title Page -....doc
^ DPSY Final Project....pdf
m
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 5 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Alasia Fuqua- Learn C = X www.awu.aleks.com F (SPAN-101-001) Beginning Spa VHL Central Sections sodium hydroxide - Google Se ickscoux/isl exe/tou IgNsiki7/8P3/H (BjuJnuCDtT6kRBabGFF3MOAKZ UVx0N202g8ikmjKtyy8Ti 165KZNcy/ErWEdBMk4J069E6ABLCdRK1ZucrG0Tdp5CNtzq?1oBw7QV O ACIDS AND BASES Predicting the relative acidity of binary acids Exanation X Check X Enter the chemical formula of a binary molecular compound of hydrogen and a Group 6A element that can reasonably be expected to be less acidic in aqueous solution than H₂Se, e.g. have a smaller K. X X + 3 3/5 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use Privacy Center Alasia B ? dla M Jan 23 * D ☐ 8:44 098 X ⠀arrow_forward5:23 PM Wed Feb 1 At a particular temperature, the solubility of CO₂ in water is 0.13 M when the partial pressure is 1.5 atm. What partial pressure (in atm) of CO₂ would give a solubility of 0.080 M? Tap here or pull up for additional resources Q @ N W # 3 F $ 4 D % 5 Question 17 of 18 ^ & 7 8 ( 9 1 4 7 +/- O 0arrow_forwardAlcoholic beverages are solutions. Typically, a beer contains about 4.50% (v/v) of ethanol fermented by yeast from sugars, while a paticular soju brand contains about 2.4% (v/v) ethanol. A korean-inspired drink was prepared by mixing 500.0 mL beer with 200.0mL of soju. The mixture was finally diluted tp 750.0mL using enough cola. a. Computes the final concentration (%w/v) of ethanol in the drink. b. How much of the drink (in mL) must a person take all at once so that the amount of ethanol ingested is 25.0mL?arrow_forward
- 5) The volume of several aqueous copper sulfate (CuSO4) solutions was precisely measured at SATP. All of the solutions were made by adding copper sulfate to 100.00 g of water. ncopper sulfate (mol) Vsolution (mL) 0 100.18 0.0330 100.16 0.0696 100.36 0.111 100.81 0.156 101.62 First, estimate the concentration-dependent partial molar volume of copper sulfate in water by taking finite differences. Second, fit the data to a hyperbola and take a derivative of the hyperbola to accurately calculate the partial molar volume of copper sulfate in water at each concentration. Interpret the partial molar volume at infinite dilution - what must copper sulfate be doing to the nearby water molecules?arrow_forwardPart A - Using the definition for mole fraction. A solution was prepared by dissolving 160.0 g of KCl in 235 g of water. Calculate the mole fraction of KCl. (The formula weight of KCl is 74.6 g/mol. The formula weight of water is 18.0 g/mol) Express the mole fraction of KCl to two decimal places. ▸ View Available Hint(s) x = Submit Η ΕΕ ΑΣΦ 0 Previous Answersarrow_forwardBaCro4 according to aleks is 1.17 x 10^-10.arrow_forward
- Rencia- Fall19- PALENCIA Activities and Due Dates> HW 15 Hint Che Resources 985/2200 core: of 22> (aq), which is used frequently in A student needs to prepare 250 mL of a 0.400 M aqueous solution of sucrose, C12H220 biological experiments. 200 mL 250 mL 150 200 100 150 50 250 mL 100 A В Question Source: MRG- General Chemistry | Publishe help terms of use contact us about us privacy policy careersarrow_forwardb Success Confirmation of Qu X < + с app.101edu.co Aktiv Chemistry X D2L Bar Graph Report - Spring x G The let-down reflex allows XCA. Fill words to blanks: Lon X Time's Up! The enthalpy of solution (AH) of KOH is -57.6 kJ/mol. If 1.56 g KOH is dissolved in enough water to make a 150.0 mL solution, what is the change in temperature (°C) of the solution? (The specific heat capacity of the solution is 4.184 J/g °C and the density of the solution is 1.02 g/mL). 1 4 7 +/- Untitled document - Google + 2 5 8 | °℃ 3 6 9 0 Update: Submit X C x 100arrow_forwardAssuming 100% dissociation, calculate the freezing point (T) and boiling point (T) of 2.05 m Na₂SO4 (aq). Colligative constants can be found in the chempendix. f Tf= Tь = psy HW 3.pdf APA Title Page....doc D PSY Final Project....pdf 'Carrow_forward
- * 00 A solution is made by mixing equal masses of methanol, CH,O, and ethanol, C, H,O. Determine the mole fraction of each component to at least three significant figures. Xmeth X eth %3D Show all 10:02 PM 10/17/2021 PrtScr Insert Delete F10 F11 F12 F8 F5 Backspace 24 6. { R. Enter H J K Shift B. N Alt Ctrl Home PgDn Endarrow_forwardHelp me solve this prelab question plzarrow_forwardA certain liquid X has a normal freezing point of 1.30 °C and a freezing point depression constant K,=7.47 °C kgmol.Calculate the freezing point of a solution made of 5.61g of alanine (C,H,NO,) dissolved in 300. g of X. -1 %3D Round your answer to 2 significant digits. 2.9 °C x10 Submit Assignment Continue O 2021 McGraw Hill LLC. All Rights Reserved. Terms of Use I Privacy Center Accessibility MacBook Air DII DD F11 F12 F9 F10 F7 F8 F6 esc F3 F4 F5 F1 F2 @ %23 $4 de 4. 6. 8. 1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY