
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Can someone help with the answer for these problems? Thank you :)
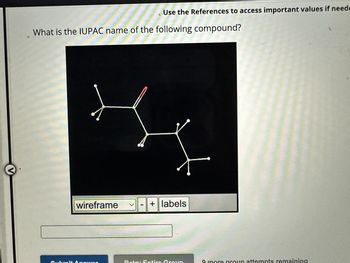
Transcribed Image Text:V
What is the IUPAC name of the following compound?
I
Use the References to access important values if neede
wireframe 1
+ labels
re
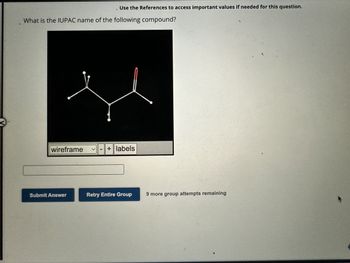
Transcribed Image Text:Use the References to access important values if needed for this question.
What is the IUPAC name of the following compound?
wireframe
Submit Answer
I
+ labels
Retry Entire Group
9 more group attempts remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 6 steps with 12 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which pesticide doesn't have a halogen functional group?ImazapyrNitisinone2,4,5-Trichlorophenoxyacetic acidSimazineAll of these have a halogenarrow_forwardNeed help ASAP!! thanks! The minimum volume of boiling water is used to dissolve 1.50 g acetanilide. The hot solution is cooled to room temperature and the acetanilide recrystallizes. What mass of acetanilide remains dissolved in water? (solubility of acetanile at 100o C is 5 g/100 mL and at room temperature is 0.54 g/100 mL). (Enter a number only with no g unit.)arrow_forwardCan i get help with this problemarrow_forward
- PURPOSE: • To synthesize esters and to identify the odor of each ● To write chemical equations for the formation of each ester APPARATUS AND MATERIALS: test tubes 400 mL beaker hotplate SAFETY: Note: ethanoic acid I;is labelled Glacial Acetic Acid PROCEDURE: Lab - MAKING ESTERS drop bottles of: Sulfuric acid is extremely corrosive. Avoid direct contact. If any touches your skin, wash it off immediately. Note the eyewash station. Alcohols and carboxylic acids are flammable. Use caution. TEST TUBE concentrated sulfuric acid methanol ethanol 1-octanol A B C D E 1. Prepare a hot water bath using the 400mL beaker and hotplate. Adjust the heating control to maintain a water temperature of about 70°C. 2. Label 5 test tubes A to F. Place the test tubes on a test tube rack 3. In the appropriate test tube, add 0.5 mL (10 drops) of alcohol followed by 0.5 mL (10 drops) of the carboxylic acid according to the table below. CARBOXYLIC ACID 0.25 g salicylic acid 0.5 mL ethanoic acid 0.5 mL butanoic…arrow_forwardDraw all alcohols with the molecular formula C,H100, making sure to avoid drawing the same alcohol twice. Editarrow_forwardI keep getting told that this product is wrong. I'm not sure what else it could be. Any help will be appreciated! Thank you!arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY