
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Hi! Please use the graph (the first pic) to answer question b (the second pic)

Transcribed Image Text:An unknown gas, D, reacts with fluorine gas to form the compound DF4(g), as represented by the following equation.
D(g) + 2 F2(9) → DF4(9)
A series of experimental trials were performed at 600°C to determine the rate law expression for the reaction. Data from the trials are shown below.
Initial Pp
Initial PF2
Initial Rate of Appearance of DF,(9)
(torr)
(torr)
(torr/min)
Trial 1
1500
1500
5.00 x 10–3
Trial 2
3000
1500
10.0 x 10-3
Trial 3
1500
3000
20.0 x 10–3
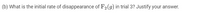
Transcribed Image Text:(b) What is the initial rate of disappearance of F2(g) in trial 3? Justify your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Your doctor prescribed eye drops for you to treat a certain condition. You obtain the eye drop liquid medication in a small bottle containing a total volume 15 ml. Your instructions were to apply 2 drops in each eye at night. Given that the volume of 1 drop is 0.05 ml. Calculate many days will the eye drop liquid medication last?Key: start by calculating how many drops are present in 1 mlarrow_forwardStep 2: Identify the dimensions of the quantities involved The second step is to identify the dimensions of the quantities involved in the problem. For example, if the problem involves distance, time, and velocity, the dimensions of these quantities would be length, time, and length/time, respectively. Step 3: Check if the units cancel out The third step is to check if the units cancel out. To do this, multiply the quantities together and check if the units cancel out, leaving only the desired unit. For example, if you are trying to find the velocity of an object and you know its distance and time, you can multiply distance by time to get velocity. If the units cancel out, you have a physically meaningful result. Step 4: Check if the result makes sense, to do this compare the units of the result with what you would expect based on the Robles statement. Using the step hints above, answer the question. You do not have to solve. Just imagine that you're teaching a friend how…arrow_forwardWhat is green chemistry? Choose the BEST answer. O Chemistry that uses only recycled chemicals. O Chemistry that makes something green in color. O Chemistry that comes from plants or uses plant materials. O Chemistry that uses non-toxic, non-harmful chemicals.arrow_forward
- Every year Every second (1 year 365 days).arrow_forwardminimum 6 sentences Future trends in green chemistry (arrow_forwardIf given a value that is plot along x-axis: 1. Find given value along x axis. 2. From this point, trace a straight line vertically (parallel to the y-axis) until it intersects with the line graph. 3. Then, trace a line horizontally (parallel to the x axis) from the intersect to the y-axis. 4. The value of y corresponding to the given x value is where the traced line intercepts with the y- axis. Based upon what you see in the graph listed below, estimate the cost of the fence installation. 450 350 A 300 4 250 200 150 100 50 10 151 20 25 30 35 Cost (in $)arrow_forward
- 1 please assist with number one in the picture. I want to be we are correct. This is not a graded question as it is a practice question . I am 60 years old and helping my son prepare for the AP exam in a few months. We do questions at the back of the textbook by Zumdahl and Zumdahlarrow_forwardDashboard My Home OWLV2 | Online teaching and learning resource from Cengag [References) A student gently drops an object weighing 15.7 g into an open vessel that is full of ethanol, so that a volume of ethanol spills out equal to the volume of the object. The experimenter now finds that the vessel and its contents weigh 10.6 g more than the vessel full of ethanol only. The density of ethanol is 0.789 g/cm°. What is the density of the object? Density = g/cm³ Submit Answer Try Another Version 10 item attempts remainingarrow_forwardSC Score: 0 of 1 point Incorrect Your answer is wrong. In addition to checking your math, check that you used the right data and DID NOT round any intermediate calculations. Steam reforming of methane (CH4) produces "synthesis gas," a mixture of carbon monoxide gas and hydrogen gas, which is the starting point for many important industrial chemical syntheses. An industrial chemist studying this reaction fills a 75.0 L tank with 14. mol of methane gas and 5.8 mol of water vapor, and when the mixture has come to equilibrium measures the amount of carbon monoxide gas to be 2.3 mol. Calculate the concentration equilibrium constant for the steam reforming of methane at the final temperature of the mixture. Round your answer to 2 significant digits. Answer Submitted: A K = 18 Submitted: Mar 2 10:23 PM Correct Answer: 0.0033 Assignments List ! F @ Review Assignment Time Spent: 39m 5s # с $ MacBook Pro G Search or type URL % & + © 2023 McGraw Hill LLC. All Rights Reserved. Terms of Use |…arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY