
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
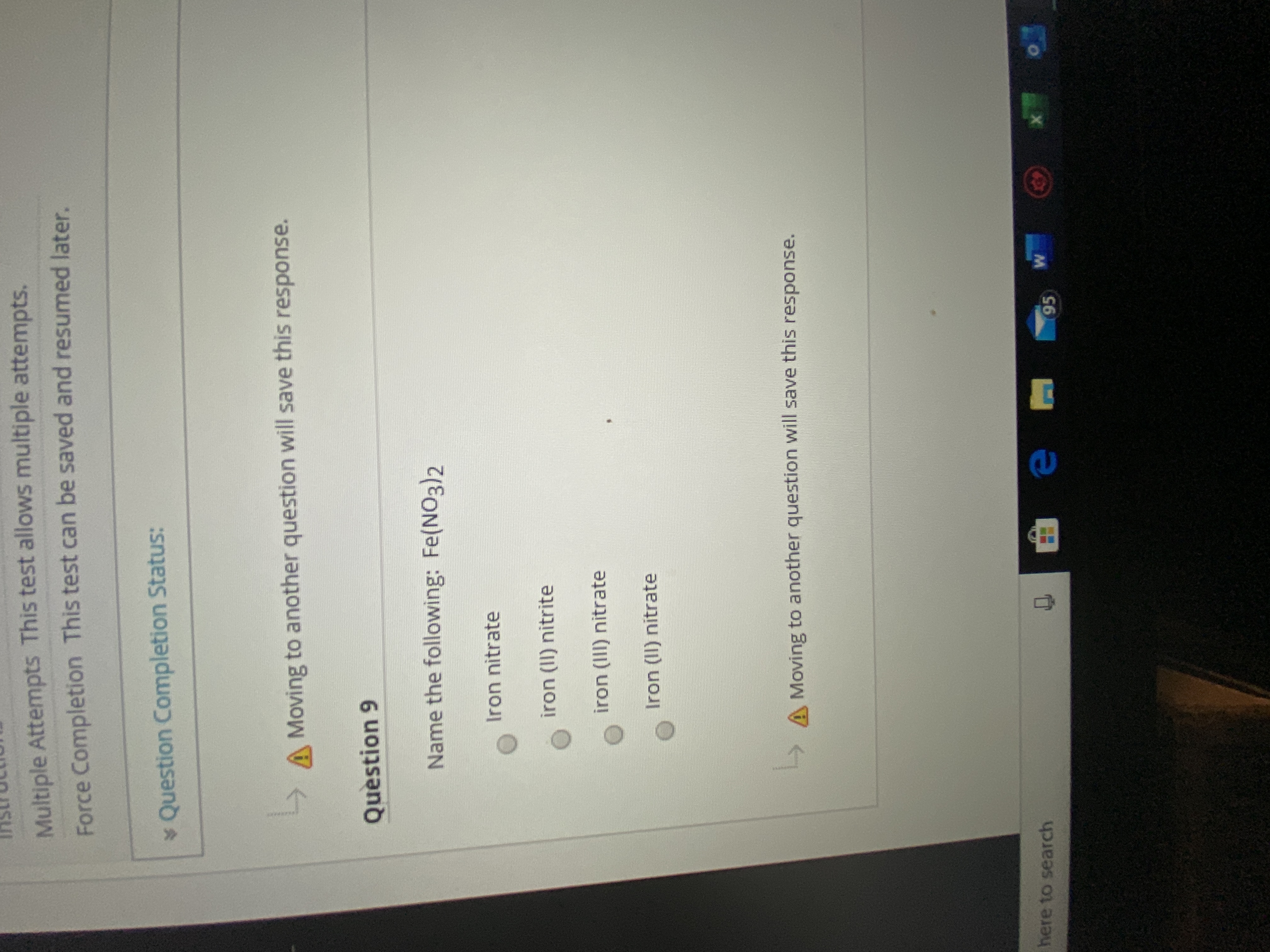
Name the following Fe(NO3)2
Transcribed Image Text:Multiple Attempts This test allows multiple attempts.
Force Completion This test can be saved and resumed later.
Question Completion Status:
A Moving to another question will save this response.
Question 9
Name the following: Fe(NO3)2
Iron nitrate
iron (II) nitrite
iron (III) nitrate
Iron (II) nitrate
Moving to another question will save this response.
here to search
95
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Fe(NH3)3 + HNO3arrow_forward(a) Flake white was a white pigment used by Renaissance artists, which was composed of a combination of lead(II) carbonate and lead(II) hydroxide. What are the correct chemical formulas for these two compounds? PbHCO3 Pb(OH)2 Pb2CO3 PbO Pb(OH)4 PbCO3arrow_forwardDetermine the charge on the transition metal cation in the following compounds. SnCl2 SnCl4 Pb(NO3)2 FeCO3arrow_forward
- Draw and label an atomic/molecular level picture of the bonding in iron (Fe) that can be used to explain iron properties (mp 1811 K,grey solid at room temp shiny,malleable,ductiles,conducts electricity)arrow_forwardWhat is the name of this ion? Cr₂O7²- Note: You must include the word "ion." CN [C=N]] cyanide ion acetate ion CH3CO₂ (or ethanoate ion) H₂C CO3²- carbonate ion hydrogen carbonate ion HCO3 (or bicarbonate ion) NO₂ nitrite ion NO₂™ nitrate ion PO43- phosphate ion HPO42- hydrogen phosphate ion H₂PO4 dihydrogen phosphate ion 0=a 0=00=4 20=20= io of OH- SO₂²- SO4 2- HSO4 C10- C10₂ C103 C104 CrO4 2- Cr₂O7²- MnO4 [O-H]] [ OS 5!!!! [c—o] %0 is I hydroxide ion sulfite ion sulfate ion hydrogen sulfate ion (or bisulfate ion) hypochlorite ion chlorite ion chlorate ion perchlorate ion chromate ion dichromate ion permanganate ionarrow_forwardWrite the formulas for the following compounds.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY