
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
See pic
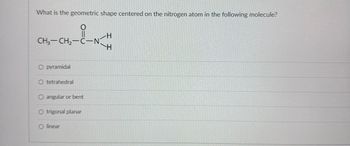
Transcribed Image Text:What is the geometric shape centered on the nitrogen atom in the following molecule?
CH3-CH2-C-N
O pyramidal
O tetrahedral
O angular or bent
O trigonal planar
Olinear
H
H
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Help plsarrow_forwardesc mb V ps lock Ft ! 1 control 141 PI Q >> A @ 2 * F2 28. NR # 80 F3 $ 888 FA % 20 FO A 244 FO & 44 F7 Aa * D'Il FB Some matches consist of a wooden stick and a head that contains tetraphosphorous trisulfide, P4S3(s), and that can be ignited on any rough surface. When the match is drawn across a rough surface, enough heat is generated to start the reaction represented by the following equation. and pur The energy that is transferred when 69.1 g of XeF4(s) is produced is DD FO Use the following information to answer the next question. Xenon tetrafluoride is a binary compound made from a noble gas. The formation of xenon tetrafluoride can be represented by the following equation. Xe(g) + 2 F₂(g) → XeF4(s) AH=-251 kJ Use the following information to answer the next two questions. DIDIOMBATILAI P4S3(s) + 8 O₂(g) → SO₂(g) A,HP,S, (S) A,HO P.O₁0(s) J F10 kJ. P4010(s) + 3 = -155.0 kJ/mol = -2 984.0 kJ/mol TULBUR di F11 F12 IN G elete 1 urn iftarrow_forwardPlease don't use hend raiting and step by step solutionsarrow_forward
- After taking multiple measurements of the same sample you have determined that the range of the sample is .06g. This range is primarily useful in determining A) Precision B) Rate of growth C) Differences between samples D) Accuracyarrow_forward10 If powdered elemental calcium and powdered elemental sodine are poured into a metal beaker and then heated stromgly, a very vigorous chemicall reaction takes place, and the Paferemces calcium iodide is formed. O element O compound O heterogeneous mixture Submit Answer Try Another Version 1 item attempt remaining Previous Ne Cengage Learning | Cengage Technical Support DELLarrow_forwardPlease correct answer and don't use hend raitingarrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY