
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
thumb_up100%
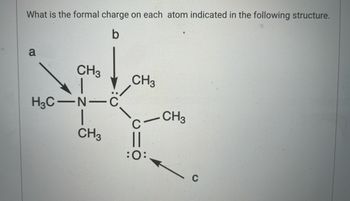
Transcribed Image Text:What is the formal charge on each atom indicated in the following structure.
b
a
CH3
H3C-N-C
CH3
CH3
||
:O:
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the formal charge on the indicated atom in the structure below. Assume all lone pairs have been shown. H C=N : -2 O-1 O (no charge) O+1 O +2 < Previous Nextarrow_forwardIdentify any formal charges in the compounds below. A) left compound B) right compoundarrow_forwardEvaluate the molecule below and assign formal charges to each atom. : : C || Cl2: 17 : Cl1 1) Formal charge of central carbon atom 2) Formal charge of oxygen 3) Formal charge of Cl1 4) Formal charge of Cl2arrow_forward
- Specify the formal charge at each of the labeled atoms, a - c, in each of the following structures. b :o: OH H H 1. b. C. +2 - +1 нн нн -1 -2 H |b c Н—С—С—С: a 2. a. b. C. Harrow_forwardAdd the missing formal chargesarrow_forwardadd formal charge where appropriate. can you also help me with the calculations? Thanksarrow_forward
- In which structure(s) below does the oxygen have a formal charge of -1? H-0-H H-ö: H-C=ö-H H3C-0-CH3 ČH3 I II III IV I only O Il only I and II I and IV O , II, and IV :0-Iarrow_forwardWhich has the shortest bond length in a series of C-H, C-C and C-O and C-N? O CH O CC O C-O O CNarrow_forward3. Keeping in mind that all row two elements need to have 8 electrons and each bond contains two electrons, draw the missing lone pair electrons on each O or N below. Then, assign the missing formal charges to each O or N. H H H H H H C H- H- C H- 中长子长 H H Harrow_forward
- 5.3 Homework Page 312- Questions 1-4 Pg 312(#1-4) Use the data from Table 1 to calculate the energy to separate 1 mol of chloromethane, CH3CI (g) , into free atoms. Ex A Edit Matharrow_forwardCalculate the formal charge for each atom that is not carbon or hydrogen in the following molecules.arrow_forwardStructure A Structure B isomers Relationship H H― C. H H + -N=N: + :N=N | H-C H | | H -H resonance structures H-N=C=N-C-H + C-NEN: H H H- C- | | H H H neither isomers resonance structures neither isomers :0: || H-C H H H -H resonance structures H H 41 41 | H H -H C=Carrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY