
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
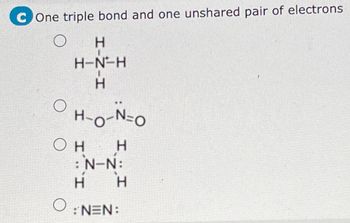
Transcribed Image Text:C One triple bond and one unshared pair of electrons
O
O
H
H-N-H
H
H-O-N=0
H
: N-N:
H H
ΟΗ
O
NEN:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What are formal charges for sulfur S and oxygen O in dimethyl sulfoxide shown below: :Ö: HC-S-C-H H HT H ΤῊ H H OS formal charge = 0; O formal charge = 0 OS formal charge = -1; O formal charge = +1 OS formal charge = +1; O formal charge = -1 S formal charge = +1; O formal charge = 0 OS formal charge = 0; O formal charge = +1 OS formal charge = 0; O formal charge = -1 OS formal charge = -1; O formal charge = 0 O None of the abovearrow_forwardIdentify the atoms in this Lewis structure. All atoms have a zero formal charge. Z- -W= N H C O A Y Z W X В Y Z X W CY W Z X D X Z W Y E W Z X Ү :<-N O O O OOarrow_forwardWhat are missing isomers and hydribizationarrow_forward
- In which structure(s) below does the oxygen have a formal charge of -1? H-0-H H-ö: H-C=ö-H H3C-0-CH3 ČH3 I II III IV I only O Il only I and II I and IV O , II, and IV :0-Iarrow_forwardWhich of the structures shown below has two sigma (o) bonds? H-C-H H-Ñ=ö: :Ci-C-Ċi: :CEO: H-N=Ö: O Cl20 O HNO O CO O CH4 HICIHarrow_forwardWhich has the shortest bond length in a series of C-H, C-C and C-O and C-N? O CH O CC O C-O O CNarrow_forward
- Choose the correct resonance hybrid for the following compound. 0 O O 49 -O-H ●ㅗㅗ 8+ 8+ HO-H co ---- 8+ 8+ H ○- + DHE O-H 8+ 8-Harrow_forward1) Classify each bond below as ionic, non-polar covalent, or polar covalent. For the polar covalent compounds, indicate the direction of the dipole. Na-Br H-CI H3C-CH,CH, H,CO-H Br-Br H-OH H,C-CF, H-CH, F-CI H,C-OH Br-Mg -Br Li-CH,CH3 2) Determine the formal charge on each of the bold atoms below. Lone pairs have been drawn in for you. Hc- ー4 H,C-cEc: Hy .C H,C CH, Hc-C OH2 H. 3) Determine the formal charge on each of the bold atoms below. You must draw in lone pairs as appropriate. H C=CH HC CH N. H. HC PH H,C CH H,C H CH, H.arrow_forwardANSWER THE FOLLOWING PROBLEMS AND EXPLAIN YOUR ANSWER FOR BETTER UNDERSTANDING. 1. Assign formal charges to each carbon, nitrogen and oxygen atom in the given species. H-C-H CH3-N=N: :OH || CH3-C-CH3arrow_forward
- Which has the shortest bond length in a series of C-H, C-C and C-O and C-N? O CH OCC O C-O C-Narrow_forward5.3 Homework Page 312- Questions 1-4 Pg 312(#1-4) Use the data from Table 1 to calculate the energy to separate 1 mol of chloromethane, CH3CI (g) , into free atoms. Ex A Edit Matharrow_forwardCalculate the formal charge for each atom that is not carbon or hydrogen in the following molecules.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY