
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
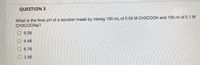
Transcribed Image Text:QUESTION 3
What is the final pH of a solution made by mixing 100 mL of 0.05 M CH3COOH and 100 ml of 0.1 M
CH3COONA?
O 5.06
O 4.46
O 6.76
O 3.56
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What is the pH of the solution after 40.5 mL of 0.200M NaOH has been added to 27.0mL of 0.300 M HCI in a titration experiment? O 2.26 O 4.56 O 6.44 O7.00arrow_forwardWhich of the following pairs would be appropriate to make a buffer of pH 8.0? O HBr and NaBr O NaHC204 and Na2C204 O HC2H3O2 and NaC2H3O2 О HСIO2 and НCІ O HBRO and NaBrOarrow_forwardA 50.00-mL solution of 0.10 M HNO2 is titrated with a 0.10 M KOH solution. After 25.00 mL of the KOH solution is added, what is the pH in the titration flask?arrow_forward
- 2helparrow_forwardIf you titrate 20.00 mL of 0.10 M HCI with 0.10 M NaOH, Calculate the pH after adding 24 mL of 0.10 M NaOH solution ? O 11.9 O 3 O 7 13.2arrow_forwardDuring a titration a 0.30 L sample of H2SO4 requires 569 mL of 2.50 M NaOH to reach the equivalence point. What is the initial concentration of H2SO4? (Do not include units in your answer. If you round during your calculation make sure to keep at least 3 decimal places. Report your answer to 2 decimal places.)arrow_forward
- Could you explain how to know which one will have a pH>7arrow_forwardWhat is the pH of an aqueous solution containing 75.0 mL of a 0.15M HNO2 solution and 45.0 mL of a .25M KNO2? What is the pH of this solution after 10.0 mL of a 0.20M solution of KOH is added? How would you categorize this problem (i.e. strong acid or weak acid titrated by strong base or weak base, buffer)?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY