
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:9
O search
X
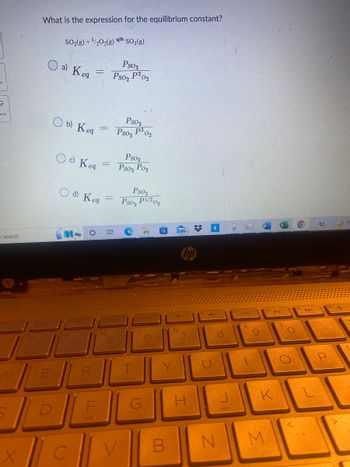
What is the expression for the equilibrium constant?
SO₂(g) + ¹/2O2(g)SO3(g)
о a) Keg
E
Ob) Keg
Od Keq
-
O d) Keg
C
R
=
6:
Ps03
Pso2 P2
Ps03
P³ 02
Ps02
02
Ps03
Pso₂ Po₂
Ps03
Pso₂ P1/202
T
G
B
H
ARUD
$ S
41
8
00
N
J
C
-
K
M
11
Expert Solution
arrow_forward
Step 1
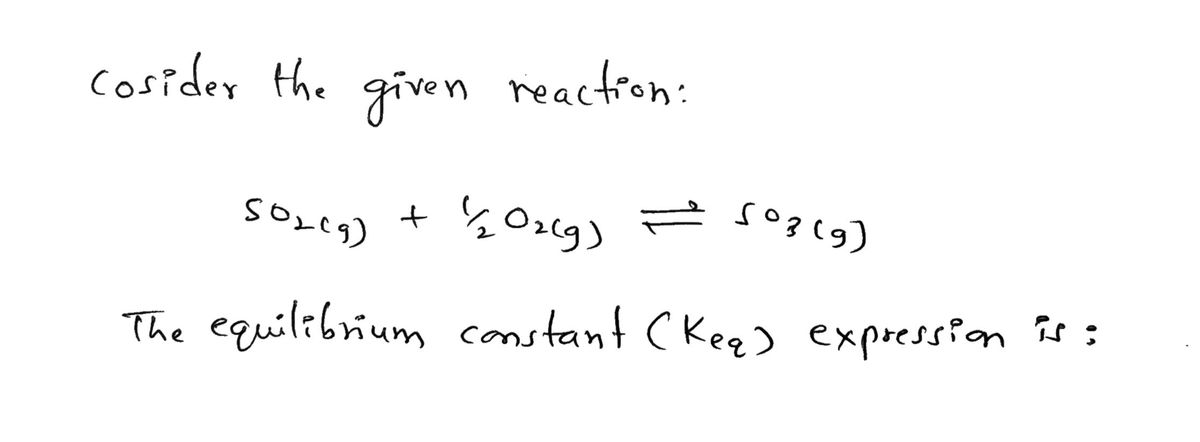
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the proper form of the equilibrium-constant expression for the equation N2 (g) + O2 (g) = 2NO(g) • View Available Hint(s) [NO] Ο Κ: [N2][O2] [NO]? O K: [N2][O2] 12 [N2][O2] O K = [NO]? 2[NO] Ο Κ- [N2][O2]arrow_forwardThe question is attached belowarrow_forwardConsider the following equilibrium: CO(g) + 3 H2(g)-CH4(g) + H2Og). If K, = 1.61 x 10-5 at 1400.0 K, calculate K. O 0.212 O 1.40 x 107 O 1.85 x 103 O 541 O 1.22 x 10-9 « Previous Nextarrow_forward
- What is the equilibrium-constant expression for the following reaction?N2O4 (g) <--> 2NO2 (g)arrow_forward-2 The equilibrium constant, K, for the following reaction is 1.71x10 at 1120 K 25O₂(g) 250₂(g) + O₂(9) Calculate K, at this temperature for the following reaction SO₂(g) SO₂(g) + 1/2O₂(g)arrow_forwardH2(g) + Br2(1) = 2HBrig) K- 4.8 x 108 Assume initial conditions of 0.400MH2lg) and excess Br (1). What is the equilibrium concentration of H2lg)? O 3.6 x 105 M O 4.9 x 1010 M O 7.5x 1010 M O 1.3x 109M O 5.3 x 10° Marrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY