
College Physics
11th Edition
ISBN: 9781305952300
Author: Raymond A. Serway, Chris Vuille
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
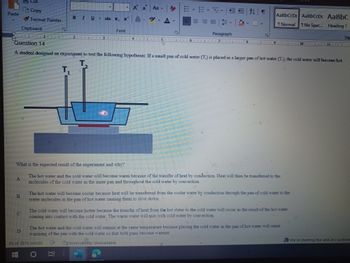
Transcribed Image Text:**Question 14**
A student designed an experiment to test the following hypothesis: If a small pan of cold water (T₂) is placed in a larger pan of hot water (T₁), the cold water will become hot.
*Diagram Explanation:*
The diagram shows a cross-section of a setup where a small pan containing cold water (T₂) is placed inside a larger pan containing hot water (T₁). Two thermometers are shown measuring the temperatures of the cold and hot water, respectively.
**What is the expected result of the experiment and why?**
A. The hot water and the cold water will become warm because of the transfer of heat by conduction. Heat will then be transferred to the molecules of the cold water in the inner pan and throughout the cold water by convection.
B. The hot water will become cooler because heat will be transferred from the cooler water by conduction through the pan of cold water to the water molecules in the pan of hot water causing them to slow down.
C. The cold water will become hotter because the transfer of heat from the hot water to the cold water will occur as the result of the hot water coming into contact with the cold water. The warm water will mix with cold water by convection.
D. The hot water and the cold water will remain at the same temperature because placing the cold water in the pan of hot water will cause warming of the pan with the cold water so that both pans become warmer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Option C was marked incorrect. Please response.
Solution
by Bartleby Expert
Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
Option C was marked incorrect. Please response.
Solution
by Bartleby Expert
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Similar questions
- Example 500 g of ice is added to an insulated cup that contains 200 g of water at 50.0°C. What is the final temperature?arrow_forwardCoefficient of linear thermal expansion is measured in the units of...choose one correct answer A. Calorie B. 1 / 0C [ 1 divided by degree celsius] C. 0C [ degree celsius] D. meterarrow_forwardChoose the most suitable definition for "heat". Select one: a. Flow of energy in structural units of chemical substances b. Energy associated with the random motion of atoms and molecules c. Energy stored in structural units of chemical substances d. Difference between the final and initial thermal energy of a systemarrow_forward
- Rubbing your hands together warms them by converting work into thermal energyIf a woman rubs her hands back and forth for a total of 20 rubs, at a distance of 7.50 cm per rub, and with an average frictional force of 40.0 N, what is the temperature increase? The mass of tissues warmed is only 0.100 kg, mostly in the palms and fingers Your answer must have three significant figures and the temperature change in centigrade .arrow_forward1 A student was cooking chiken in the frying pan below. What transfer of heat between the pan and the chicken is responsible for cooking the chicken? A radiation B convection Ce lectrical conductionarrow_forwardQuestion 2arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning
College PhysicsPhysicsISBN:9781305952300Author:Raymond A. Serway, Chris VuillePublisher:Cengage Learning University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON
University Physics (14th Edition)PhysicsISBN:9780133969290Author:Hugh D. Young, Roger A. FreedmanPublisher:PEARSON Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press
Introduction To Quantum MechanicsPhysicsISBN:9781107189638Author:Griffiths, David J., Schroeter, Darrell F.Publisher:Cambridge University Press Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning
Physics for Scientists and EngineersPhysicsISBN:9781337553278Author:Raymond A. Serway, John W. JewettPublisher:Cengage Learning Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley
Lecture- Tutorials for Introductory AstronomyPhysicsISBN:9780321820464Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina BrissendenPublisher:Addison-Wesley College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON
College Physics: A Strategic Approach (4th Editio...PhysicsISBN:9780134609034Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart FieldPublisher:PEARSON

College Physics
Physics
ISBN:9781305952300
Author:Raymond A. Serway, Chris Vuille
Publisher:Cengage Learning

University Physics (14th Edition)
Physics
ISBN:9780133969290
Author:Hugh D. Young, Roger A. Freedman
Publisher:PEARSON

Introduction To Quantum Mechanics
Physics
ISBN:9781107189638
Author:Griffiths, David J., Schroeter, Darrell F.
Publisher:Cambridge University Press

Physics for Scientists and Engineers
Physics
ISBN:9781337553278
Author:Raymond A. Serway, John W. Jewett
Publisher:Cengage Learning

Lecture- Tutorials for Introductory Astronomy
Physics
ISBN:9780321820464
Author:Edward E. Prather, Tim P. Slater, Jeff P. Adams, Gina Brissenden
Publisher:Addison-Wesley

College Physics: A Strategic Approach (4th Editio...
Physics
ISBN:9780134609034
Author:Randall D. Knight (Professor Emeritus), Brian Jones, Stuart Field
Publisher:PEARSON