
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Transcribed Image Text:you need extra neip.
Good luck!
Your progress has been saved
Question 5 of 10
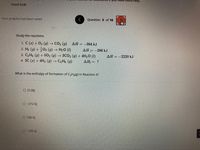
Study the reactions.
1. C (s) + O2 (9) → CO, (g)
2. H2 (9) +02 (g) → H2O (1)
3. C3 Hs (g) + 502 (g) → 3CO2 (g) + 4H2O (1)
4. 3C (s) + 4H2 (g) C3 Hs (g)
AH = -394 kJ
AH = -286 kJ
AH = –2220 kJ
AH; = ?
What is the enthalpy of formation of C3H3(g) in Reaction 4?
O 212k)
O-212 k)
O 106 kJ
2-106 kJ
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Amounts of reactants used and products obtained Molar Mass Volume used Mass used or Moles used or Density (g/mL) (g/mol) (mL) produced (g) produced (mol) 100.158 0.96 Cyclohexanol 1.50 82.143 0.105 Cyclohexene Product theoretical yield (g) Product percent yield (%)arrow_forwardBalance the following chemical equation and determine the lowest whole numbered coefficient for H2O Al4C3(s)+H2O(l)——>CH4(g)+Al(OH)3(s)arrow_forwardT Ma M PA USN Be * Ma E Ad G ph ph Do E Un O Ac. 6 Pec O Na O Pe Со Bas 3 Pe Stu "app.101edu.co Question 11 of 11 What is the mass in grams of H2 that can be formed from 55.8 grams of NH3 in the following reaction? 2 NH:(g) → 3 H:(g) + N:(g)arrow_forward
- A household water heater containing 50 gallons (227 L) of water is heated burning natural gas (methane, molar mass = 16.043 g mol-1) via the following reaction: CH4(g) + O2(g) CO2(g) + H20(O A,H = -891 kJ mol-1 What mass of methane, in units of g, is needed to heat the 227 L of water in the heater from 25.0°C to 44.0°C? Assume: • the heater is 100% efficient in its use of heat energy • no heat is lost to the surroundings specific heat of water is: 4.184 Jg-1 °C-1 density of water is: 0.9970 g mL¯1arrow_forwardBalance the cquation C,H18() + 0>(g)→ CO,(g) + H;O(g) CO,(g) + H,0(g)arrow_forwardBalance the following equation. What is the sum of the coefficients in the balanced equation? KCIO;(s) + P(s) - P,O10(s)+ KCI(s)arrow_forward
- FeSO4 + Potassium phosphate à Iron (II) phosphate + K2SO4 State the Molar Ratio of the reaction.arrow_forward3. Determine the enthalpy change (AH₂) for the final reaction below. a. CO(g) + ½ O₂(g) → CO₂(g) AH, -67.6 kcal b. N₂(g) + O₂(g) →→→ 2NO(g) AH₂ = 43.2 kcal c. CO(g) + NO(g) →→→ CO₂(g) + ¹½ N₂(g) AH₂ = ?arrow_forwardComplete the reactions below with appropriate reaction conditions, make sure that your set of reaction conditions produce the indicated product as a major product. Please indicate clearly whether the reaction conditions occur in one or several distinct steps. Reaction Conditions Reactant Product a) Br b) OH c) HOarrow_forward
- Your lab partner accidentally mixed some sodium chloride with your sample of Epsom salts (MgSO₄・7H₂O). You want to make a standard solution of magnesium ion, and this is the only sample of a magnesium salt you have. To determine the amount of magnesium salt in the mixture, you heat 100.00 g to drive off the water of hydration and find that the anhydrous mixture has a mass of 57.75 g. How many grams of the original salt mixture must you add to 1.000 L of water to make a 0.100 M solution of Mg²⁺ ion?arrow_forwardThe psychoactive drug methamphetamine ("speed"), which is sold as the prescription medication Desoxyn, C10H15N, under- goes a series of reactions in the body; the net result of these reac- tions is the oxidation of solid methamphetamine by oxygen gas to produce carbon dioxide gas, liquid water, and an aqueous solution of urea, CH4N₂O. Write the balanced equation for this net reaction.arrow_forwardHow many moles of CO2 are produced when 0.735 moles of isopropanol are burned?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY