
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
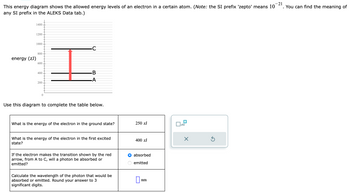
Transcribed Image Text:This energy diagram shows the allowed energy levels of an electron in a certain atom. (Note: the SI prefix 'zepto' means 10-21. You can find the meaning of
any SI prefix in the ALEKS Data tab.)
energy (zJ)
1400
1200
1000
800
600
400
200
0
I
B
-A
Use this diagram to complete the table below.
What is the energy of the electron in the ground state?
What is the energy of the electron in the first excited
state?
If the electron makes the transition shown by the red
arrow, from A to C, will a photon be absorbed or
emitted?
Calculate the wavelength of the photon that would be
absorbed or emitted. Round your answer to 3
significant digits.
250 ZJ
400 ZJ
absorbed
emitted
nm
x10
X
S
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A certain shade of blue has a frequency of 7.25 × 1014 Hz. What is the energy of exactly one photon of this light? Planck's constant is h = 6.626 × 10-34 J.s. E = Jarrow_forwardA particular form of light is found to have a wavelength of 459 nm. Calculate the frequency of this light in Hz. (1 Hz = 1/s)arrow_forwardA photon of light with a wavelength of 200.0 nm strikes a metal surface and an electron is ejected with a measured velocity of 7.26 x 105 m/s. Given the following table of threshold energies, identify the metal. Metal Copper Lead Gold Platinum Threshold Energy 7.53 x 10-19 J 6.63 x 10-19 J 8.17 x 10-19 J 9.05 x 10-19 J Lead Gold Copper Platinumarrow_forward
- An electron absorbs a single photon with a wavelength = 127 nm. Calculate the energy of the photon.arrow_forwardPayalbenarrow_forwardA certain shade of blue has a frequency of 7.04 x 10¹4 Hz. What is the energy of exactly one photon of this light? Planck's constant is h 6.626 x 10-34 J.s. = E = Jarrow_forward
- 8. If you have light that travels with a frequency of 9x1012 Hz. What is the energy of this light?arrow_forwardThe human eye is a complex sensing device for visible light. The optic nerve needs a minimum of 2.0 × 101' J of energy to trigger a series of impulses that eventually reach the brain. How many photons of blue light (475 nm) are needed?arrow_forwardConvert between number of photons and energy given a particular wavelength. Low level light therapy (LLLT) using RED light was observed to promote wound healing. The daily dose provided was 24 J/cm. Given the wavelength of 635 nm, how many photons were required to deliver the dose to a 1 cm? area?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY