
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
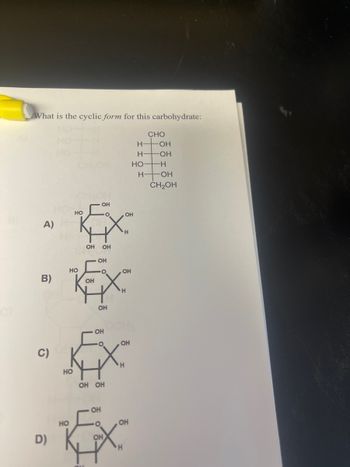
Transcribed Image Text:What is the cyclic form for this carbohydrate:
CHO
OH
HO
O
OH
A)
H
OH
OH
OH
HO
°
OH
B)
OH
H
OH
OH
°
OH
C)
H
HO
OH OH
D)
HO
OH
OH
OH
H
HOH
HOH
HO -H
H-
-OH
CH2OH
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 3 images

Knowledge Booster
Similar questions
- Q3: Use arrows to match the terms (A) column with (B) column. Some may be used more than once and others not at all. 1- Tirglyceride 2- Glycogen 3- Sphingomyelins 4- Membranes 5- Starch 6- Proteins 7- Cholesterol 8- Lactose B a- contain glycerol backbone b- glucose é- amino acids d- esters of fatty acids e- octapeptide f- disaccharide g- polysaccharide K- compound lipids i- steroid-like j- lipids k- polar lipids + glucose storage in animals 9- Waxes 10AngiotensinlIarrow_forwardwhat type of saccharide is this molecule?arrow_forwardPlease don't provide handwritten solution.arrow_forward
- Are digitoxin / digoxin composed of one type or more than one type of monosaccharide? How are the monosaccharide derivatives of digitoxin / digoxin classified (alcohol sugar, deoxy sugar, amino sugar, carboxylic acid sugar)? Are the monosaccharides furanose or pyranose rings?arrow_forwardProvide the systematic name of the cyclic monosaccharide shown here Be sure to include the structural form as a component of the name. H Question 78 of 95 OH CH₂OH H OH H mann H OH O B- D- a- L- fruct gluc pyran furan galact ose oside ol OH Harrow_forwardSC ||| Identifying the parts of a disaccharide Take a look at this molecule, and then answer the questions in the table below it. CH₂OH 1 H OH H OH H -O OH. H H O Explanation 2 @ H Is this a reducing sugar? CH₂OH OH H H W Does this molecule contain a glycosidic bond? If you said this molecule does contain a glycosidic bond, write the symbol describing it. 。 H If you said this molecule does contain a glycosidic bond, write the common names (including anomer and enantiomer labels) of the molecules that would be released if that bond were hydrolyzed. If there's more than one molecule, separate each name with a comma. OH Check #3 OH E $ 4 R % 5 - 20 T O yes 6 Ono O yes O no 0-0 MacBook Pro 0 Y & 7 α X +00 * В © 2023 McGraw Hill LLC. All Rights Reserved. 8 ローロ S 9arrow_forward
- O triglyceride Question 7 1 pts Classify the following lipid (choose all that apply). CH3(CH2)6-0–ĉ-(CH2)10CH3 O triglyceride O monounsaturated O cis alkene(s) O wax ester O polyunsaturated O fatty acid O steroid O saturated O trans alkene(s) 1 pts Question 8 Lr APR étv 16 口arrow_forward49. The following compound is a(n) нонно H,N-C-ċ-Ѭċ-ċ-OH H;NCH;CH;CH;CH; H A. monosaccharide B. monopeptide C. disaccharide D. dipeptide E. diglyceridearrow_forwardCellulose is not digestible by humans because it contains glucose units linked by -glycosidic bonds. O a-1,6 O a-1,4 O a-1,2 O B-1,4 O B-1,2arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY