
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
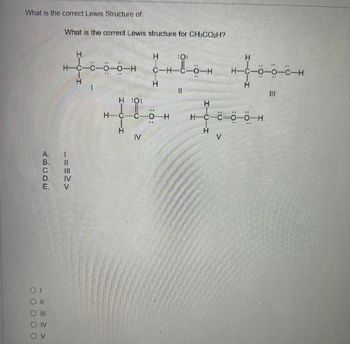
Transcribed Image Text:What is the correct Lewis Structure of
១០០០u
E.
01
Oll
O III
OIV
OV
What is the correct Lewis structure for CH3CO₂H?
H▬C▬C▬Ö▬Ö▬H
===>>
..
H :O:
H-C
IV
H :O:
|||
C-HC-O-H
-O-H
11
H
H
V
H
H-C-C-0-0-H
HORS
H-C-0-0-C-H
H
1
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 6 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If both could be answered that would be great!arrow_forwardO ||| crosoft C W W Microsoft esc Microsoft 6.52.210... ~ Calculating formal charge A student proposes the following Lewis structure for the ozone (03) molecule. V :0-0-0 Assign a formal charge to each atom in the student's Lewis structure. atom central O left O ! 1 Explanation right O formal charge 2 0 U 0 Check 9,002 > a # 3 X DEC 8 $ 4 G E buty % 5 tv ♫ A 6 7 2022arrow_forwardWhich of these pairs are resonance structures? i. ii. 道. H H-C H H H H-C–C–C—C—H H H H H H | H H 一 H H H–C–C H H-C H H H H C H 大学 6: H :OH O:H H:O: H H :Ö: H C-H OH :0₁arrow_forward
- O A student proposes the following Lewis structure for the carbon dioxide (CO₂) molecule. osoft C W W Microsoft :01C=0: Assign a formal charge to each atom in the student's Lewis structure. Microsoft 6.52.210... esc atom left O C right O Explanation 1 Q A formal charge 2 U U U Check 8,999 W S 280 Q # 3 E X 17 D DEC 8 4 5 R do LO % bartleby 5 LL tv ♫ NIN T 6 G Y & 2022 McGraw Hill L 7 H * 00 U 8 LEarrow_forwardEthyl acetate, C4H8O2, is a fragrant substance used both as a solvent and as an aroma enhancer. Its Lewis structure is (Figure 1). Figure 1 of 1 H O: H H H-C-C-0-C-C-H нн Type here to searcharrow_forwardQUESTION 3 What is the electronic configuration for the magnesium ion 1s2 2s2 2p63s2 01s2 2s2 2p6 e1s2 2s22p4 1s2 2s22p63s1 C152 2s2 2p63s22p QUESTION 4 Rank the indicated C-C bonds in increasing order of bond length.arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY