
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
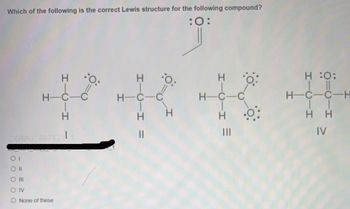
Transcribed Image Text:Which of the following is the correct Lewis structure for the following compound?
:0:
.O.
H-C- C-C
O III
OIV
OGMU 887121
HICIH
None of these
H-C-C
H
||
O.
H
H-C-C
H
H :O:
H-C-C-H
HH
IV
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- O ELECTRONIC STRUCTURE AND CHEMICA... Deciding whether a Lewis... Decide whether these proposed Lewis structures are reasonable. proposed Lewis structure [O=C-H]* :0: : CIC CI: [¤¤-6: 0/5 Is the proposed Lewis structure reasonable? Yes. No, it has the wrong number of valence electrons. The correct number is: 0 No, it has the right number of valence electrons but doesn't satisfy the octet rule. 000 The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: 0 Alia V No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* Yes. No, it has the wrong number of valence electrons. The correct number is: No, it has the right number of valence electrons but doesn't satisfy the octet rule. The symbols of the problem atoms are:* X Ar If two or more atoms of the same element don't satisfy the octet rule, just enter the chemical symbol as many times as necessary. For example,…arrow_forwardplease write the number that corresponds to the correct Lewis structure for the molecule shown in the box below. H H H-C-C-Ö-Ö-H_C-H-C-Ö-H H structures? :O: H C-H-C-O-HH-C-Ö-Ö-C-H || CH3CO₂H ܪܬܐܒܪܘܘܢ || H :O: | || H-C-C-O-H ||| IV H-C-C=Ö-Ö-H write the numbers of the following bond-line structures that represent identical V IVarrow_forwardDraw a Lewis structure for the given skeletal molecule. Note: Multiple bonds and lone pairs are not shown in the skeletal molecule. H. H H HHH | | | + H-C-C-N-H II H H I C-H How many lone pairs of electrons are found in the Lewis structure of your molecule?arrow_forward
- Which of the following Lewis structures is incorrect? ☐ H-N-H H О :F-F: О H H-C-H H ☐ H_O_H H + N=Narrow_forwardDecide whether the Lewis structure proposed for each molecule is reasonable or not. molecule proposed Lewis structure OF 2 BeH₂ PF4 || :O: H - Be H F - P F W ... :F: :F: Is this a reasonable structure? If not, why not? Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: O Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: O Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: 0 * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as…arrow_forwardEvery atom in the molecule below shows a correct bonding pattern (free electron pairs are not shown). The molecule also contains at least 2 Cs and at bond: // H-C-C | ОН O True O False エーOーエarrow_forward
- Chem. 065 Ionic and covalent bonds 1. Complete the following worksheet: Compound Ionic bond or covalent bond: which applies NO CrO CaCO3 PC13 NaCl CS₂ 2. Complete the following work sheet Compound Number of Number of Number of triple bonds single bonds double bonds HCN CHCI, -2 SO₂-² SO₂ HCOCI CO₂ SC₁₂ CIO, Number of lone Molecular pair of shape electrons on the central atom.arrow_forwardLook at the molecule depicted below, several atoms need more or less electrons to complete its octet. Select all that apply from the list below. :F: H3C-Ċ- | | : F :0 -H The O with a double bond needs two electrons. the C with a double bond needs to lose two electrons. The H needs two electrons. The O with two single bonds needs two electrons. F needs two more electrons. O The C with four bonds needs two electrons. :o:arrow_forwardDecide whether the Lewis structure proposed for each molecule is reasonable or not. molecule proposed Lewis structure 2- So 1 13 OC1₂ : 0: :0 :0: IS || :0: 12- [-1-1] :O: :C1=0=C1: Is this a reasonable structure? If not, why not? Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: Yes, it's a reasonable structure. No, the total number of valence electrons is wrong. The correct number is: No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times…arrow_forward
- Decide whether the Lewis structure proposed for each molecule is reasonable or not. proposed Lewis structure Is this a reasonable structure? If not, why not? molecule O Yes, it's a reasonable structure. O No, the total number of valence electrons is wrong. NH, H N=H The correct number is:| No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: LI Yes, it's a reasonable structure. :F: O No, the total number of valence electrons is wrong. BF, The correct number is: :F-B –F: O No, some atoms have the wrong number of electrons around them. The symbols of the problem atoms are: U Yes, it's a reasonable structure. F No, the total number of valence electrons is wrong. The correct number is: IF No, some atoms have the wrong number of electrons around them. .. F: The symbols of the problem atoms are: U * If two or more atoms have the wrong number of valence electrons around them, just enter the chemical symbol for the atom as many times as…arrow_forwardEthyl acetate, C4H8O2, is a fragrant substance used both as a solvent and as an aroma enhancer. Its Lewis structure is (Figure 1). Figure 1 of 1 H O: H H H-C-C-0-C-C-H нн Type here to searcharrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY