
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
What is the configuration of this chiral center?
R - configuration
S - configuration
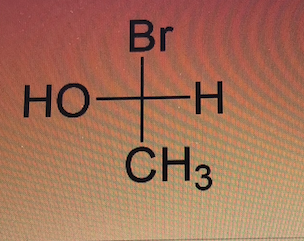
Transcribed Image Text:Br
НО-
HO-H
CH3
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Is SOCIBr a chiral molecule? Yes Noarrow_forwardWhich is true about monosaccharides? The linear form is more prevalent and contains a carbonyl The linear form is more prevalent and contains a carbonyl The cyclic form is more prevalent and contains a carbonyl The cyclic form is more prevalent and does not contain a carbonylarrow_forwardConsider the aldohexose (monosaccharide) structure. Which of the following correctly designates the absolute configuration (R or S) of each chiral carbon in this molecule? HO shown below in a line OH OH OH OHarrow_forward
- Shown below is 3-fluoro-2-butanol. What is the configuration of the chiral centers? OH H. F *CH3 CH3 O 1. The configuration is (25,35). O 2. The configuration is (2R,3R). O 3. The configuration is (25,3R). O 4. The configuration is (2R,35).arrow_forwardHow many chiral carbons are present in lysine (aqueous form)? zero chiral carbons one chiral carbon two chiral carbons three chiral carbons four chiral carbonsarrow_forwardWhat are the enantiomers of ibuprofen? Point out the chiral centers and indicate absolute configuration for each chiral center.arrow_forward
- Draw the Haworth projection of the disaccharide made by joining two D-glucose molecules with an a(1→6) glycosidic bond. If the disaccharide has more than one anomer, you can draw any of them. Click and drag to start drawing a structure. × Śarrow_forwardDo enantiomers have the same boiling point or melting point?arrow_forwardDraw a structure for a singly substituted chiral chloroalkane that contains four carbon atoms. Indicate the chirality center with an asterisk (*).arrow_forward
- Using wedge-and-dash bonds, modify the bonds on the chiral carbon in the molecule below so the molecule has S stereochemical configuration. H₂C- HO Ć Ć X C+arrow_forwardNumber of chiral center/s and stereocenters of the product formedarrow_forwardJiguall how many chiral carbon in this compound MEDIAarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY