
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
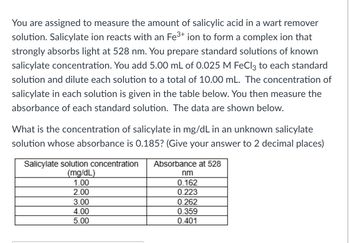
Transcribed Image Text:You are assigned to measure the amount of salicylic acid in a wart remover
solution. Salicylate ion reacts with an Fe³+ ion to form a complex ion that
strongly absorbs light at 528 nm. You prepare standard solutions of known
salicylate concentration. You add 5.00 mL of 0.025 M FeCl3 to each standard
solution and dilute each solution to a total of 10.00 mL. The concentration of
salicylate in each solution is given in the table below. You then measure the
absorbance of each standard solution. The data are shown below.
What is the concentration of salicylate in mg/dL in an unknown salicylate
solution whose absorbance is 0.185? (Give your answer to 2 decimal places)
Salicylate solution concentration Absorbance at 528
(mg/dL)
1.00
2.00
3.00
4.00
5.00
nm
0.162
0.223
0.262
0.359
0.401
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- If your first titration yielded a concentration of 0.4 mg/mL and your second titration yielded a concentration of 0.54 mg/mL, what was the average concentration for your two titrations (in mg/mL)? Include the unit and two decimal places in your answer.arrow_forwardPart1: 1.05×10-1 m glucose solution made by dissolving the glucose in 100.0 kg of water: ____ mol Part2: 2.35×10-2 m Na2CrO4 solution made by dissolving the Na2CrO4 in 1000.0 g of water:____ molarrow_forwardA Moving to another question will save this response. Question 20 Some graduate students have a solution of 1 M NaCl in the lab. What volume of the NaCl solution and what votume of water do they need to mix together to make 1 L of 0.035 M NaC? O 35 mL of 1M NaCl and 35 mL of H20 O 35 ml of 1M NaCl and 965 mL of H20 O 35 mL of 1M NaCl and 65 mL of H20 O 3.5 mL of 1M NaCl and 6.5 mL of H20 A Moving to another question will save this response. K< Qu MacBook Pro 46 888 F4 V8 FS F3 & %23 24 3 4. T K V. B F. 1R C. E. S'arrow_forward
- A packet of Emergen-C contains 1.000 x 103 mg of vitamin C, also known as ascorbic acid. If this packet were dissolved in 0.25 L of water, what would be the molarity of ascorbic acid in the solution? The molar mass of ascorbic acid is 176.124 g/mol. 1.4x10-3 M 23 М 700 M 2.3x10-2 M 4.0 M Submit Request Answerarrow_forward14. The concentration of the original unknown protein solution is determined to be 0.2173 mg/mL. What is the protein concentration in the units of parts-per-million (ppm)? One ppm = 1 mg/L. 0.217 4602 217.3 217arrow_forward1. What percent solution if 11.2 g of Na2SO4 is dissolve to make 112 g of solution? Answer to 2 decimal place 2. The concentration of sugar in a soft drink is measured to be 8.60%. How many grams of sugar are in 268 g of the drink? Answer to 1 decimal place 3. If you have 3.40 g of NaOH solid, what mass of solution can be made to 0.32 % concentration? Answer to 1 decimal place 4. What mass of KF is need to prepare 440 mL of 0.12 M solution? Answer to 2 decimal places. Include the unit. 5. The concentration of sugar in a soft drink is measured to be 9.2 %. How many grams of sugar are in 196 g of the drink? Answer to 1 decimal place 6. What mass of KNO3 is need to prepare 626 mL of 0.24 M solution? Answer to 2 decimal places. Include the unit. 7. What is the molarity if 886 g of (NH4)2CO3 to make 1,716 mL of solution? Answer to 2 decimal places. Answer to 2 decimal places.arrow_forward
- You you have a very concentrated solution (12 M) of potassium chloride (KCl). You need it to be at the lowest concentration possible for the experiment you are about to conduct. The problem is you forgot to order lab supplies, so you only have 2 L of distilled water left. What would be the final concentration if you added the 2 L of distilled water to the 0.5 L of 12 M KCl?arrow_forwardA dopamine vial that contains 200 mg in 5 mL is diluted with 0.9% sodium chloride to prepare a total volume of 500 mL. What is the concentration (in micrograms per mL) of dopamine within the 500 mL?arrow_forwardUse the graph on the last page to answer questions 10, 11 and 12. At 80˚C, what mass of sodiumchloride dissolves in1.0 L of water?arrow_forward
- How many of the data sets listed could be used to find the number of grams of solute in solution? 34567 27 g of unknown compound in water ( Compound is Soluble) A 0.05 M MgCl2 solution x 1022 molecules of unknown compound in water A 1.2 L solution of MgCl2 with 0.05 M Mg²+ A 1.2 L solution of MgCl2 A 1.2 L solution of unknown compound with 0.05 M Cl- 15 x 1022 molecules of nonelectrolyte CH3OH in waterarrow_forwardUse the solubility graph to answer the following question. Name the type of solution if 750 g of NaNO3 was placed in 600 mL of the water at 50 °C. Solubility Curves KI Solubility (g/L of solvent) 1400 1300 1200 1100- 1000 900 800 700- 600 500 400 300 200 100 0 polyunsaturated unsaturated saturated 0 supersaturated -HCI saturated with excess solute SO₂ NaNO3 -KNO₂ NHẠCH KCI -KCIO3 NaCl NH3 10 20 30 40 50 60 70 80 90 100 Temperature (°C)arrow_forwardA small portion (2.22 mL) of ascorbic acid solution (0.2310 mg/L) was transferred to a volumetric flask. Then, water was added up to the 100.0 ml mark of the volumetric flask (ie, the final volume of the diluted solution was 100.0 mL) Calculate the concentration (in mg/L) of the diluted ascorbic acid solution.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY