
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
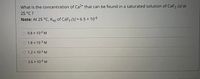
Transcribed Image Text:What is the concentration of Ca2+ that can be found in a saturated solution of CaF2 (S) at
25 °C ?
Note: At 25 °C, Ksp of CaF2 (S) = 6.5 × 10-6
%3|
9.8 x 10-2 M
1.8 x 10-3 M
1.2 x 10-2 M
2.6 x 10-3 M
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution
Trending nowThis is a popular solution!
Step by stepSolved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Answer the following questionsarrow_forwardStep by Step. Find the Value of the Equilibrium constant (K) at 300K for the following reaction. The value for Kpat 300 K is 1.5 x 10-5/atm2. N4(g) +4 H2(g) 4 NH2arrow_forwardThe value of Ksp for PbCrO4 (s) in equilibrium with water at 25 °C is 2.8 × 10-13. Calculate the solubility of PbCrO4 (s) in water at 25 °C in grams per liter.arrow_forward
- 1. What is the equilibrium constant expression for the following reaction? (Pay careful attention to the phase indicators!) 2 HCl (g) + Mg(OH)2 (s) ? MgCl2 (aq) + 2 H2O (l)arrow_forwardthanks for the help for reviewarrow_forwardThe solubility product for silver chloride is 1.6 x 10−10. What is the molar solubility of silver chloride in a 6.5 x 10−3 M AgNO3 aqueous solution at 298 K? Group of answer choices 6.5 x 10−3 M 2.5 x 10−8 M 1.3 x 10−5 M 4.8 x 10−4 M 2.8 x 10−20 Marrow_forward
- The equilibrium constant, Keq, for the reaction: 2 NOCl(g) -> 2NO(g) + Cl2(g) is 2.4 x 10-7 (no units). What is the Keq for the reaction 1/3 Cl2(g) + 2/3 NO (g) -> 2/3 NOCl(g)arrow_forward5. Calculate the solubility (g/L) for silver sulfate, Ag2SO4 in 0.250 M AgBr. Ksp = 1.5 x 10s %3Darrow_forwardAt a certain temperature the value of the equilibrium constant, Kc, is 0.8375 for the reaction. SiO2 (g) + 4HCI (g) = SiCl4 (g) + 2H2O (g) What is the value of Kc for the following reaction? 2SiCl4 (g) + 4H20 (g) 2SiO2 (g) + 8HCI (g) B 1.426 -0.8375 -1.6750 Show work..don't give Handwritten answer. D 0.8375 £0.7014 F-0.7014 G-1.426 0.9152arrow_forward
- Mass, Volume and Keq An equilibrium mixture, at 712°C in a 1972-mL container, involving the chemical system N2(g) + 3H2(g) — 2NH3(g) is found to contain 1.26 g of №₂, 0.227 g of H₂, and 9.76×10-³ g of NH3. Calculate the equilibrium constant (Keq expressed in terms of the molar concentrations) at the given temperature. (No units required.) 0.0148arrow_forwardConsider the following equilibrium reaction: PBr5(g) + 1/2 O2(g) = POB13(g) + Br2(g) Kc = 5.6 x 102 at 512 K Calculate K. for the reaction 2 PBr5(g) + O2(g) = 2 POBR3(g) + 2 Br2(g) at the same temperature. 24 1.1 x 103 280 5.6 x 102 O None of these 3.2 x 105arrow_forwardDecide whether each of the following statements is true or false. If false, change the wording of the statement to make it true. a) Only the concentration of CO2 appears in the equilibrium expression for the reaction: CaCO3 (s) ↔ CaO (s) + CO2 (g). B) For the reaction CaCO3 (s) ↔ CaO (s) + CO2 (g), the value of K is numerically the same whether the amount of CO2 is expressed as molarity or as gas pressure.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY