
Chemistry: The Molecular Science
5th Edition
ISBN: 9781285199047
Author: John W. Moore, Conrad L. Stanitski
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
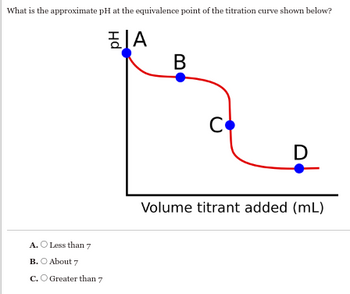
Transcribed Image Text:What is the approximate pH at the equivalence point of the titration curve shown below?
A
B
A. O Less than 7
B. About 7
C. Greater than 7
C
D
Volume titrant added (mL)
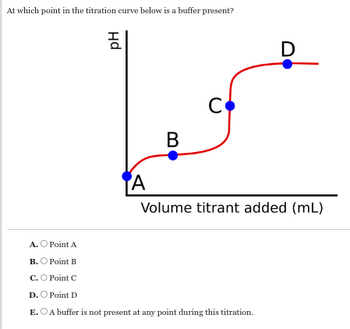
Transcribed Image Text:At which point in the titration curve below is a buffer present?
Hd
HO
D
A
B
C
Volume titrant added (mL)
A. Point A
B. Point B
C. O Point C
D. O Point D
E.O A buffer is not present at any point during this titration.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps

Knowledge Booster
Similar questions
- The weak base ethanolamine. HOCH2CH2NH2, can be titrated with HCl. HOCH2CH2NH2(aq)+H3O+(aq)HOCH2CH2NH3+(aq)+H2O(l) Assume you have 25.0 mL of a 0.010 M solution of ethanolamine and titrate it with 0.0095 M HCl. (Kb for ethanolamine is 3.2 107.) (a) What is the pH of the ethanolamine solution before the titration begins? (b) What is the pH at the equivalence point? (c) What is the pH at the halfway point of the titration? (d) Which indicator in Figure 17.11 would be the best choice to detect the equivalence point? (e) Calculate the pH of the solution after adding 5.00, 10.0, 20.0, and 30.0 mL of the acid. (f) Combine the information in parts (a), (b), (c), and (e), and plot an approximate titration curve.arrow_forwardWhen 40.00 mL of a weak monoprotic acid solution is titrated with 0.100-M NaOH, the equivalence point is reached when 35.00 mL base has been added. After 20.00 mL NaOH solution has been added, the titration mixture has a pH of 5.75. Calculate the ionization constant of the acid.arrow_forwardThe titration of 0.100 M acetic acid with 0.100 M NaOH is described in the text. What is the pH of the solution when 35.0 mL of the base has been added to 100.0 mL of 0.100 M acetic acid?arrow_forward
- A 0.2481 M solution of KOH is used to titrate 30.00 mL of 0.269 M hydrobromic acid. Assume that volumes are additive. (a) Write a balanced net ionic equation for the reaction that takes place during the titration. (b) What are the species present at the equivalence point? (c) What volume of KOH is required to reach the equivalence point? (d) What is the pH of the solution 1. before any KOH is added? 2. halfway to the equivalence point? 3. at the equivalence point?arrow_forwardA buffer solution with it pH of 12.00 consists of Na3PO4 and Na2HPO4. The volume of solution is 200.0 mL. (a) Which component of the buffer is present in a larger amount? (b) If the concentration of Na3PO4 is 0.400 M, what mass of Na2HPO4 is present? (c) Which component of the buffer must be added to change the pH to 12.25? What mass of that component is required?arrow_forwardA buffer solution is prepared by dissolving 1.50 g each of benzoic acid, C6H5CO2H, and sodium benzoate, NaC6H5CO2, in 150.0 mL of solution. (a) What is the pH of this buffer solution? (b) Which buffer component must be added, and in what quantity, to change the pH to 4.00? (c) What quantity of 2.0 M NaOH or 2.0 M HCl must be added to the buffer to change the pH to 4.00?arrow_forward
- Cyanic acid (HOCN) is a weak acid with AL, = 3.5 X IO-4. Consider the titration of 25.0 inL of 0.125 M HOCN with 0.125 M NaOH. Calculate the pH of the solution at each of the following points. Before any NaOH has been added. .After 12.5 mL of NaOH has been added. After 23.0 inL of NaOH has been added. .After 27.0 mL of KOH have been added. Use your calculator or a spreadsheet to plot the titration curve, and use your graph to estimate the pH at the equivalence point.arrow_forwardMarble is almost pure CaCO3. Acid rain has a devastating effect on marble statuary left outdoors. Assume that the reaction which occurs is CoCO3(s)+ H+(aq)Ca2+(aq)+HCO3(aq) Neglecting all other competing equilibria and using Tables 15.1 and 13.2, calculate (a) K for the reaction. (b) the molar solubility of CaCO3 in pure water. (c) the molar solubility of CaCO3 in acid rainwater with a pH of 4.00.arrow_forwardA 0.4000 M solution of nitric acid is used to titrate 50.00 mL of 0.237 M barium hydroxide. (Assume that volumes are additive.) (a) Write a balanced net ionic equation for the reaction that takes place during titration. (b) What are the species present at the equivalence point? (c) What volume of nitric acid is required to reach the equivalence point? (d) What is the pH of the solution before any HNO3 is added? (e) What is the pH of the solution halfway to the equivalence point? (f) What is the pH of the solution at the equivalence point?arrow_forward
- Consider the nanoscale-level representations for Question 111 of the titration of the aqueous strong acid HA with aqueous NaOH, the titrant. Water molecules and Na+ ions are omitted for clarity. Which diagram corresponds to the situation: (a) After a very small volume of titrant has been added to the initial HA solution? (b) Halfway to the equivalence point? (c) When enough titrant has been added to take the solution just past the equivalence point? (d) At the equivalence point? Nanoscale representations for Question 111.arrow_forwarda Draw a pH titration curve that represents the titration of 50.0 mL of 0.10 M NH3 by the addition of 0.10 M HCl from a buret. Label the axes and put a scale on each axis. Show where the equivalence point and the buffer region are on the titration curve. You should do calculations for the 0%, 30%, 50%, and 100% titration points. b Is the solution neutral, acidic, or basic at the equivalence point? Why?arrow_forwardYou are given the following acidbase titration data, where each point on the graph represents the pH after adding a given volume of titrant (the substance being added during the titration). a What substance is being titrated, a strong acid, strong base, weak acid, or weak base? b What is the pH at the equivalence point of the tiration? c What indicator might you use to perform this titration? Explain.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning