
Chemistry
10th Edition
ISBN: 9781305957404
Author: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Question
Transcribed Image Text:LJ Labflow - Course: UN
N MyLab and Mastering x
Course Home
WA HW 3.6 - Math 181 - S X
b Answered: Calculate th x
Sy derivative of x^{7cos( X
A openvellum.ecollege.com/course.html?courseld=16418591&OpenVellumHMAC=27f8d06b346b59fd4ealae7a332a17d9#10001
KPropiem set 5 (Chapter 5) Adaptive Foilow-up
Course Home
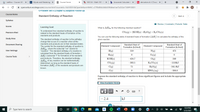
Standard Enthalpy of Reaction
Item 4
Syllabus
II Review | Constants | Periodic Table
Scores
Learning Goal:
What is AHm for the following chemical reaction?
To understand how standard enthalpy of reaction is
related to the standard heats of formation of the
CO2 (g) + 2KOH(s)→H2O(g) +K2CO3 (s)
Pearson eText
reactants and products.
You can use the following table of standard heats of formation (AH:) to calculate the enthalpy of the
Study Area
The standard enthalpy of reaction is the enthalpy
change that occurs in a reaction when all the
reactants and products are in their standard states.
The symbol for the standard enthalpy of reaction is
AHn. where the subscript "rxn" stands for
"reaction." The standard enthalpy
calculated from the standard heats of formation (
AH:) (subscript "f" for formation) of its reactants
and products. Therefore, the standard enthalpy
AHm of any reaction can be mathematically
determined, as long as the standard heats of
formation (AH;) of its reactants and products are
given reaction.
Document Sharing
Standard Heat of
Standard Heat of
Element/ Compound
Element/ Compound
Formation (kJ/mol)
Formation (kJ/mol)
User Settings
of a reaction is
H(g)
218
N(g)
473
H2(g)
O2 (g)
Course Tools
>
KOH(s)
-424.7
O(g)
249
CO2 (g)
-393.5
K2CO3 (s)
-1150kJ
C(g)
71
H2O(g)
-241.8kJ
known
C(s)
HNO3 (aq)
-206.6
Express the standard enthalpy of reaction to three significant figures and include the appropriate
units.
View Available Hint(s)
?
2.4
kJ
D Pearson
9:24 PM
P Type here to search
3/22/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by stepSolved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- A scientist measures the standard enthalpy change for the following reaction to be -57.0 kJ:Ca(OH)2(aq) + 2 HCl(aq)CaCl2(s) + 2 H2O(l)Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of H2O(l) is ___ kJ/mol.arrow_forwardA 25.0 mL portion of dilute HCl (aq) is combined with a 25.0 mL portion of dilute NaOH in a coffee-cup calorimeter. Both solutions are initially at a temperature of 23.3 ºC. The reaction produces enough heat to raise the final temperature of the 50.0 mL of liquid in the calorimeter to 26.8 ºC . What is qrxn in J?arrow_forwardUse the table below of enthalpies of formation to determine the heat of the following reaction. Fe2O3(s) + 13CO(g) → 2Fe(CO)5(g) + 3CO2(g) Substance Molar Enthalpy of Formation (kJ/mol) Fe2O3(s) -824.2 CO(g) -110.52 Fe(CO)5(g) -773.9 CO2(g) -393.51arrow_forward
- A cubic piece of platinum metal (specific heat capacity = 0.1256 J/°C・g) at 200.0°C is dropped into 1.00 L of deuterium oxide ('heavy water,' specific heat capacity = 4.211 J/°C・g) at 25.5°C. The final temperature of the platinum and deuterium oxide mixture is 42.9°C. The density of platinum is 21.45 g/cm³ and the density of deuterium oxide is 1.11 g/mL. What is the edge length of the cube of platinum, in centimeters?arrow_forwardA scientist measures the standard enthalpy change for the following reaction to be 208.5 kJ :CH4(g) + H2O(g)3H2(g) + CO(g)Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of CH4(g) is kJ/mol.arrow_forwardPotassium nitrate, KNO 3, has a molar mass of 101.1 g/mol. In a constant-pressure calorimeter, 41.8 g of KNO3 is dissolved in 277 g of water at 23.00 °C. KNO3(s) H₂O K+(aq) + NO3(aq) The temperature of the resulting solution decreases to 20.90 °C. Assume that the resulting solution has the same specific heat as • water, 4.184 J/(g · °C), and that there is negligible heat loss to the surroundings. How much heat was released by the solution? Isoln = What is the enthalpy of the reaction? AH rxn = KJ kJ/molarrow_forward
- Use the References to access important values if needed for this question. Using standard heats of formation, calculate the standard enthalpy change for the following reaction. Fe,O3(s) + 3H,(g)2Fe(s) + 3H,0(g)arrow_forwardConsider the following reaction. CH3OH(g) CO(g) + 2 H2(g) H = +90.7 kJ (a) Is the reaction exothermic or endothermic? endothermicexothermic (b) Calculate the amount of heat transferred when 40.0 g of CH3OH(g) are decomposed by this reaction at constant pressure.H = kJ(c) If the enthalpy change is 11.0 kJ, how many grams of hydrogen gas are produced? g(d) How many kilojoules of heat are released when 13.5 g of CO(g) reacts completely with H2(g) to form CH3OH(g) at constant pressure?H = kJ(e) Calculate E when 360.0 g of CH3OH(g) completely reacts at a constant temperature of 300 K and constant pressure of 0.95 atm. R = 8.314 J/mol*K and R = 0.08206 atm*L/mol*K kJ HopHelpCh5N1arrow_forwardConsider the following reaction. CH3OH(g) CO(g) + 2 H2(g) H = +90.7 kJ (a) Is the reaction exothermic or endothermic? exothermic endothermic (b) Calculate the amount of heat transferred when 55.0 g of CH3OH(g) are decomposed by this reaction at constant pressure.H = kJ(c) If the enthalpy change is 11.0 kJ, how many grams of hydrogen gas are produced? g(d) How many kilojoules of heat are released when 12.0 g of CO(g) reacts completely with H2(g) to form CH3OH(g) at constant pressure?H = kJ(e) Calculate E when 450.0 g of CH3OH(g) completely reacts at a constant temperature of 300 K and constant pressure of 0.95 atm. R = 8.314 J/mol*K and R = 0.08206 atm*L/mol*Karrow_forward
- A scientist measures the standard enthalpy change for the following reaction to be 209.4 kJ : CHĄ(g) + H20(g)3H2(g) + CO(g) Based on this value and the standard enthalpies of formation for the other substances, the standard enthalpy of formation of CH4(g) is kJ/mol.arrow_forwardA cubic piece of platinum metal (specific heat capacity = 0.1256 J/°C・g) at 200.0°C is dropped into 1.00 L of deuterium oxide ('heavy water,' specific heat capacity = 4.211 J/°C・g) at 25.5°C. The final temperature of the platinum and deuterium oxide mixture is 35.1°C. The density of platinum is 21.45 g/cm³ and the density of deuterium oxide is 1.11 g/mL. What is the edge length of the cube of platinum, in centimeters?arrow_forwardConsider the following reaction. CH3OH(g) CO(g) + 2 H2(g) H = +90.7 kJ ((b) Calculate the amount of heat transferred when 40.0 g of CH3OH(g) are decomposed by this reaction at constant pressure.H = kJ(c) If the enthalpy change is 20.0 kJ, how many grams of hydrogen gas are produced? g(d) How many kilojoules of heat are released when 12.5 g of CO(g) reacts completely with H2(g) to form CH3OH(g) at constant pressure?H = kJ(e) Calculate E when 880.0 g of CH3OH(g) completely reacts at a constant temperature of 300 K and constant pressure of 0.95 atm. R = 8.314 J/mol*K and R = 0.08206 atm*L/mol*K kJ HopHelpCh5N1arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY